Water

If a person knows nothing else about chemistry, he or she will likely know that water is H 2 O. The chemical formula for water is common knowledge, used in advertisements, elementary school science classes, and casual conversation. But more than just a conversation piece, the formula H 2 O can tell a chemist a great deal of information.
For starters, H 2 O indicates that water is composed of two hydrogen atoms and one oxygen atom. That this is so can be demonstrated using very simple apparatus—a couple of pieces of wire, a battery, and some tap water. Electrolysis—the decomposition of water molecules with electricity—will result when the wires are connected to the ends of the battery and the other ends are immersed in the water with a small gap between them. One electrode releases bubbles of pure oxygen and the other pure hydrogen. Measuring the volume of gases released reveals that twice as much hydrogen is produced as oxygen. Twice as much gas means two hydrogen atoms for every one oxygen atom. Of course, this is our modern understanding of water. When these experiments were first tried, around 1800, an explanation for the results was not available. But the experiments did force scientists to think about the nature of water.
From our modern understanding of both the formula of water and the Periodic Table, we also know that the hydrogen atoms in water are bound to the oxygen. That is, water is "HOH" and not "HHO." We know that atoms can form either covalent or ionic bonds to give molecules. In water, the interaction of hydrogen and oxygen is a polar covalent bond, meaning that the two elements share a pair of electrons and that each atom contributes one of the electrons in the pair. Since hydrogen is the first element of the Periodic Table, it has only one electron and can form just one covalent bonding interaction. In the case of water, hydrogen bonds by sharing its electron with the oxygen. If hydrogen shared its electron with the other hydrogen atom in this instance, there would be no electron available to interact with the oxygen. Indeed, hydrogen gas, H 2 , results when two hydrogen atoms form a covalent bond, and hydrogen gas is very different from water.
In a pure (nonpolar) covalent bond, both atoms have possession of the electron pair exactly the same amount of time. In a polar covalent bond, there is unequal sharing that results from an inequity in the distribution of the electrons due to the effective nuclear charge on the atoms. This polarization of the O-H interaction is critical to explaining all of the properties of water. It results in water having a dipole with the hydrogens having a slight positive charge and the oxygen having a slight negative charge. (More precisely, the advanced explanation is that the molecular orbital that describes the oxygen-hydrogen interaction has more oxygen character, resulting in a skewed electron distribution.)
If we consider oxygen's position in the Periodic Table, we know that it starts with six valence electrons, and since it has two bonds with hydrogen, two of its electrons are involved in bonding pairs. This means that the oxygen has four electrons remaining. These electrons are organized into two "non-bonding" pairs. That is, the oxygen of water has four pairs of electrons around it—two that are interacting in polar covalent bonds with hydrogen and two that are not interacting when water is in the gaseous state. Four electron pairs means that the atoms adopt a tetrahedral arrangement with the two hydrogens occupying two corners and the electron pairs occupying the other two.
In a perfect tetrahedron, the angle between the hydrogens would be 109.5°, but because the lone pairs occupy a little more space, the experimentally measured angle in water is actually 104.5°.
The presence of two lone pairs plays a very important role in "hydrogen bonding," which is one of the most critical properties of water. The positively charged hydrogen of one water molecule can be attracted to the lone pair of an adjacent molecule, resulting in a weak hydrogen bonding interaction. This bonding is much weaker than the polar covalent bond that holds a water molecule together, but it is a substantial inter-molecular interaction resulting in two water molecules being attracted to one another. Water forms an extended network of hydrogen bonding interactions, with each water molecule capable of both creating and accepting two hydrogen bonds. As a result each and every water molecule in the liquid or solid state is surrounded by four hydrogen bonded neighbors. The presence of hydrogen bonding interactions means:
- that water has an anomalously high melting and boiling point;
- that solid water or ice is less dense than liquid; and
- that water has a high surface tension.
Melting and Boiling Point of Water
Phase changes in matter result because of a change in the translational motion of molecules. A solid is a solid because its molecules are stuck in place. In a liquid, molecules can move past one another but are still closely associated. In a gas, molecules move independent of one another and only occasionally collide. It would make sense then that lighter molecules would shift from a solid to a liquid to a gas at lower temperatures than heavy molecules because they require less energy to get moving. Consider the molecular substances in Table 1.
Hydrogen gas, being the lightest and smallest molecule in the list, has the lowest melting point ( −259°C or 14.15 K or −434°F) and boiling point ( −253°C or 20.15 K or −423°F). Similarly, of the second row compounds with hydrogen, methane (CH 4 ) has the lowest melting and boiling points. However, water does not follow this trend. Its melting point is 0°C or 32°F. Its boiling point is 100°C or 212°F. Compared to the other molecules around it or its heavier cousin, hydrogen sulphide (H 2 S), water has melting and boiling points that are anomalously high. This is due to the fact that the hydrogen bonds between water molecules must be broken for a phase transition to occur. The extra energy required results in more heat being necessary and a higher temperature.
Density of Ice
Hydrogen bonding interactions between water molecules hold the molecules in place in the solid state. The O-H .... O interaction spaces all of thewater molecules in an orderly array, much like students sitting in rows of desks. This spacing provides an open structure. When water is in the liquid state, the water molecules hold on to each other through hydrogen bonding interactions, but individual molecules can occupy the space between rows. The result is that at a molecular level, more liquid water molecules can occupy a given volume than when water is in the solid state. More students will fit in a classroom if they are allowed to stand than if they are arranged in nice neat rows. More molecules or more mass in a given volume means a higher density.
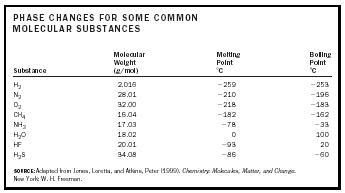
| PHASE CHANGES FOR SOME COMMON MOLECULAR SUBSTANCES | |||
| Substance | Molecular Weight (g/mol) | Melting Point ˚C | Boiling Point ˚C |
| SOURCE: Adapted from Jones, Loretta, and Atkins, Peter (1999). Chemistry: Molecules, Matter, and Change. New York: W. H. Freeman. | |||
| H 2 | 2.016 | −259 | −253 |
| N 2 | 28.01 | −210 | −196 |
| O 2 | 32.00 | −218 | −183 |
| CH 4 | 16.04 | −182 | −162 |
| NH 3 | 17.03 | −78 | −33 |
| H 2 O | 18.02 | 0 | 100 |
| HF | 20.01 | −93 | 20 |
| H 2 S | 34.08 | −86 | −60 |
The decrease in density between water and ice has a number of important implications for the world around us. Ice floats because it is less dense than liquid water. This is not true of any other liquid/solid equilibrium. Solid methane sinks in liquid methane and solid ammonia sinks in liquid ammonia. Floating ice means that ponds and lakes freeze from the top down, allowing fish and other biota to live protected from the cold weather of winter. If water froze from the bottom up, life as we know it would not have evolved on Earth.
Surface Tension
Surface tension is a bulk property of matter and results in liquid water trying to contract to the smallest possible surface area for a given volume. Surface tension explains why water beads up on the surface of a freshly waxed car and droplets of water in a fog are spherical. The sphere is the shape with the minimum area for a given volume. Surface tension results because of the asymmetry of forces at the surface of liquid water. Water molecules at the surface are missing their hydrogen bonding interactions on one side. They are being "tugged" back into the bulk of solution.
Of course, occasionally water molecules have sufficient energy to leave the surface, resulting in evaporation. Conversely, sometimes water molecules in the gaseous state strike the surface of a drop of water and have insufficient energy to leave again. The result is condensation. The competing rates of evaporation and condensation lead to the formation of clouds and fog, to cloudy mirrors after a shower and iced-up windows on a winter's day.
Surface tension is also critical to capillary action. A surface that is covered in suitable molecules or functional groups, such as a glass surface or the cellulose of paper, will interact with water molecules and can actually draw the molecules out of the bulk. In this case, the interaction with the surface is stronger than the hydrogen bonding interaction between adjacent water molecules. As a result, water will creep up a glass tube or adsorb into paper. The latter is critically important in mopping up a spill or mess.
Universal Solvent
Water is often called the "universal solvent," as it is capable of dissolving a wide range of compounds—from sugars to salt, from DNA to hydrogen. Again, hydrogen bonding interactions play a role. For example, sugar dissolves because of the hydrogen bonding interactions between the hydroxyl groups on the sugar molecules (-OH groups) and the water molecules. But of equal importance to the dissolution of substances in water is water's capacity to act as a dipole. Water's negatively charged oxygen binds to sodium ions in salt while the positively charged hydrogens interact with the chloride ions. The result is that sodium chloride or table salt dissolves into ionic species that are more energetically stable with the sodium and chloride ions surrounded by water.
The ability of water to dissolve a wide variety of substances makes it the ideal medium for living organisms. Water's great solvency is also the reason that water pollution is so pervasive. Almost any substance will dissolve in water, including pesticides, herbicides, industrial waste, household byproducts, and a wide variety of other potentially harmful compounds. Indeed, we rely on the dissolving properties of water to get our clothes clean.
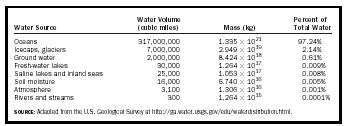
| Water Source | Water Volume(cubic miles) | Mass (kg) | Percent of Total Water |
| SOURCE: Adapted from the U.S. Geological Survey at http://ga.water.usgs.gov/edu/waterdistribution.html. | |||
| Oceans | 317,000,000 | 1.335 10 21 | 97.24% |
| Icecaps, glaciers | 7,000,000 | 2.949 10 19 | 2.14% |
| Ground water | 2,000,000 | 8.424 10 18 | 0.61% |
| Fresh-water lakes | 30,000 | 1.264 10 17 | 0.009% |
| Saline lakes and inland seas | 25,000 | 1.053 10 17 | 0.008% |
| Soil moisture | 16,000 | 6.740 10 16 | 0.005% |
| Atmosphere | 3,100 | 1.306 10 16 | 0.001% |
| Rivers and streams | 300 | 1,264 10 15 | 0.0001% |
But the dirt and grime from our clothes must end up somewhere, and that somewhere is in the water that we discharge from our homes. The Law of Conservation of Matter says that matter can be neither created nor destroyed. The atoms and molecules that we dissolve into the water in our washing machines are only being removed to another location.
Dealing with the pollution of water is a huge task, and for too long the philosophy was "the solution to pollution is dilution." Dilution is no longer an acceptable approach, as it just shifts the problem instead of addressing it. Significant effort is being spent in both addressing the real problems of water pollution and in ensuring that we have access to clean water sources. There are many techniques for purifying water, with distillation providing the cleanest and purest water. Unfortunately, distillation requires a lot of energy as it is difficult to overcome the hydrogen bonds between water molecules. Distillation also leaves behind the polluting material, which must be disposed of in a manner that does not allow it to come in contact with water and thus simply dissolve again. The difficulties of maintaining clean water is one of the major challenges facing us in the twenty-first century. For without water, life as we know it would not exist. It is because of the shape and the interactions of that very simple molecule, H 2 O, that water is the most essential of all chemical compounds.
SEE ALSO Green Chemistry ; Molecular Geometry ; Valence Bond Theory ; Water Pollution ; Water Quality .
Todd W. Whitcombe
Bibliography
Atkins, Peter W. (1987). Molecules. New York: Scientific American Library.
Leopold, Luna B.; Davis, Kenneth S.; and the Editors of Life (1966). Water. New York: Time Incorporated New York.
Stanitski, Conrad L.; Eubanks, Lucy Pryde; Middlecamp, Catherine H.; and Pienta, Norbert J. (2003). Chemistry in Context : Applying Chemistry to Society. New York: McGraw-Hill.
Internet Resources
United States Geological Survey Water-Resources Investigations Report 98–4086. "Water Science for Schools." Available from http://ga.water.usgs.gov/edu/index.html .