Joseph John Thomson
ENGLISH PHYSICIST 1856–1940
Joseph John Thomson, always known as "J. J.," was born in Manchester, England, on December 18, 1856. His fame derives primarily from his discovery of the electron in 1897. He studied physics and mathematics, first in Manchester, and in 1876 went to Trinity College, Cambridge University, and never left. He graduated in 1880 and in 1884 succeeded Lord Rayleigh as professor of physics and director of the Cavendish Laboratory. (When he retired from Cavendish in 1919 he passed the baton to Ernest Rutherford). Thomson made Cambridge a world center for atomic physics. He won the Nobel Prize for physics in 1906 for his work on the electron, and seven of his research associates went on to win Nobel Prizes. The electron could almost be said to have been a family heirloom, as his son, George Paget Thomson, won the Nobel Prize for physics (in 1937) for showing the wave nature of the electron.
His early work in electromagnetism led him to say, in 1893: "There is no other branch of physics which affords us so promising an opportunity of penetrating the secret of electricity." He turned his attention to cathode rays, and his subsequent investigations of these rays led him to the idea that they consisted of bodies smaller than atoms. Thomson's main contribution to science was the clear identification of the electron and its characterization as an elementary, subatomic particle in 1897. He showed that cathode rays were deflected by both magnetic and electric fields, and he was able to measure a cathode ray's charge/mass ( e/m ) ratio. Figure 1 is a schematic of diagram of his apparatus, showing how a beam of electrons can be subjected to opposing electric and magnetic fields, which can be adjusted until their effects balance. This enabled him to estimate the mass of the electron as 1/1,837 of a hydrogen atom. The electron was the first subatomic particle to be discovered, and he made the inspired guess that it was a universal constituent of matter. He said: "… [W]e have in the cathode rays matter in a new state, a state in which the subdivision of matter is carried very much
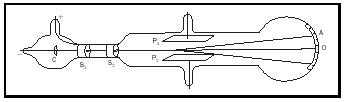
further than in the ordinary gaseous state: a state in which all matter … is of one and the same kind; this matter being the substance from which all the chemical elements are built up." He announced his discovery in the course of a public lecture at the Royal Institution in London, on April 30, 1897, in which he said: "Could anything at first sight seem more impractical than a body which is so small that its mass is an insignificant fraction of the mass of an atom of hydrogen?" Thomson referred to electrons as "corpuscles" (even in his 1906 Nobel lecture).
Thomson devised the famous plum pudding model of the atom, in which electrons were compared to negative plums embedded in a positively charged pudding. The idea was wrong, and his successor at Cambridge, Ernest Rutherford, was soon to develop the nuclear model of the atom.
Thomson investigated positive rays, which consist of ionized atoms, beginning in 1906. He was able to use a combination of electric and magnetic fields to separate different charged atoms of elements on the basis of their charge/mass ratios. He was the first to show that neon contained two atoms of slightly different masses, in a paper published in 1913. As part of the conclusion of the paper he wrote: "There can, therefore, I think, be little doubt that what has been called neon is not a simple gas but a mixture of two gases, one of which has an atomic weight about 20 and the other about 22. The parabola due to the heavier gas is always much fainter than that due to the lighter, so that probably the heavier gas forms only a small percentage of the mixture." The two forms of neon were called isotopes by Frederick Soddy. One of Thomson's students, Frederick Aston, developed Thomson's idea of multiple species of an element, and in 1919 Aston produced the first mass spectrograph (an instrument that determined isotopic ratios), ancestor of today's mass spectrometer.
Thomson was a great advocate of pure research, in contrast to applied research, declaring: "[R]esearch in applied science leads to reforms, research in pure science leads to revolutions, and revolutions, whether political or industrial, are exceedingly profitable things if you are on the winning side." Thomson was knighted in 1908 and received many awards and honors. He died during the early part of World War II, on August 30, 1940, and is buried in Westminster Abbey near Sir Isaac Newton, in recognition of his great contributions to science.
SEE ALSO Magnetism ; Spectroscopy .
Peter E. Childs
Bibliography
Dahl, Per F. (1997). Flash of the Cathode Rays: A History of J. J. Thomson's Electron. Philadelphia: Institute of Physics Pub.
Falconer, Isobel (1997). "J. J. Thomson and the Discovery of the Electron." Physics Education 32(4): 226–231.
Gerward, Leif (1997). "The Discovery of the Electron." Physics Education 32(4): 219–225.
Thomson, J. J. (1936). Recollections and Reflections. London: G. Bell and Sons.
Internet Resources
Articles about Thomson. Cavendish Laboratory. Available from http://www.phy.cam.ac.uk/cavendish/history/ .
"The Discovery of the Electron." American Institute of Physics. Available from http://www.aip.org/history/electron/ .
"Information on the Electron." Science Museum of London. Available from http://www.iop.org/Physics/Electron/ .
Thomson, J. J. Articles. Available from http://webserver.lemoyne.edu/faculty/giunta .
Thomson, J. J. "Carriers of Negative Electricity." Nobel Lecture. Nobel e-Museum. Available from http://www.nobel.se/physics/laureates/ .
Comment about this article, ask questions, or add new information about this topic: