Tellurium

MELTING POINT:
449.5°C
BOILING POINT:
990°C
DENSITY:
6.25 g/cm
3
MOST COMMON IONS:
Te
2−
,
Te
2
2−
,
Te
4+
,
TeO
5
2−
,
TeO
4
2−
Tellurium was discovered in gold ores by Franz Joseph Müller von Reichenstein, the chief inspector of mines in Transylvania (Romania), in 1782. Tellurium was named, however, by M. Klaproth, who continued Müller von Reichenstein's work and isolated the element in 1798. Its name originates from the Latin tellus , which means "earth."
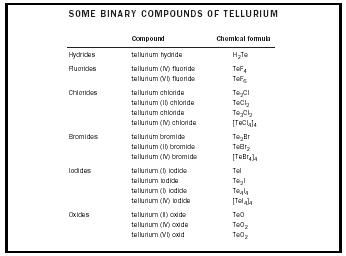
| SOME BINARY COMPOUNDS OF TELLURIUM | ||
| Compound | Chemical formula | |
| Hydrides | tellurium hydride | H 2 Te |
| Fluorides | tellurium (IV) fluoride | TeF 4 |
| tellurium (VI) fluoride | TeF 6 | |
| Chlorides | tellurium chloride | Te 2 Cl |
| tellurium (II) chloride | TeCl 2 | |
| tellurium chloride | Te 3 Cl 2 | |
| tellurium (IV) chloride | [TeCl 4 ] 4 | |
| Bromides | tellurium bromide | Te 2 Br |
| tellurium (II) bromide | TeBr 2 | |
| tellurium (IV) bromide | [TeBr 4 ] 4 | |
| Iodides | tellurium (I) iodide | TeI |
| tellurium iodide | Te 2 I | |
| tellurium (I) iodide | Te 4 I 4 | |
| tellurium (IV) iodide | [TeI 4 ] 4 | |
| Oxides | tellurium (II) oxide | TeO |
| tellurium (IV) oxide | TeO 2 | |
| tellurium (VI) oxid | TeO 2 | |

Tellurium is a comparatively rare element, is seventy-third in order of crustal abundance (approximately 0.001 ppm), and is occasionally found as the native metal . Many of its minerals occur together with the sulfides of chalcophilic metals (e.g., Cu, Ag, Au, Zn, Cd, Hg, Fe, Co, Ni, Pb, As, Bi). It is obtained from anode slime produced in the electrolytic refining of Cu. Tellurium has eight naturally occurring isotopes ; the most abundant ones are 130 Te (33.8%) and 128 Te (31.7%). Crystalline tellurium has a silvery-white appearance and exhibits a metallic luster but is usually obtained as a dark gray powder. It is brittle and easily pulverized. Tellurium is a p-type semiconductor and shows varying conductivity with crystal alignment (its electrical resistivity is approximately 1 ohm cm at 25°C). Its conductivity increases slightly with exposure to light. It can be doped with silver, copper, gold, tin, and other elements. Other nuclear and physical properties include: atomic radius: 160 pm; ionic radius: 221 pm (Te 2− ); 97 pm (Te 4+ ); 56 pm (Te 6+ ); and ionization energy: 869 kJ/mol.
The main uses of tellurium are: as semiconductors; in the formation of alloys with lead (to prevent corrosion), cast iron (to improve machinability), copper, and stainless steel; in ceramic materials; tinting glass.
Bibliography
Emsley, John (1989). The Elements. New York: Oxford University Press.
Greenwood, N. N., and Earnshaw, A. (1984). Chemistry of the Elements. New York: Pergamon Press.
Internet Resources
WebElements. Available from http://www.webelements.com .
Comment about this article, ask questions, or add new information about this topic: