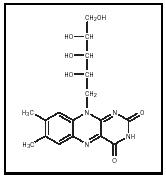
Riboflavin
Riboflavin, also known as vitamin B 2 , gets its name from its sugar alcohol (ribitol), and from its yellow color and its fluorescence under UV light ( flavin comes from the Latin word for yellow). Its systematic names are 7,8-dimethyl-10-(D-ribo-2,3,4,5-tetrahydroxypentyl)isoalloxazine and 7,8-dimethyl-10-ribitylisoalloxazine; its formula is C 17 H 20 N 4 O 6 . Riboflavin has a molar mass of 376.37 grams (13.3 ounces). It is heat-stabile but easily degraded by light. Riboflavin was referred to as vitamin G in the early part of the twentieth century because it was recognized as a dietary factor needed for growth. Riboflavin was first isolated in 1879, and its chemical structure was determined in 1933.
As determined by the National Research Council of the National Academy of Sciences, the recommended daily allowance (RDA) of riboflavin for adults is about 1.5 milligrams (5.3 × 10 −5 ounces). The amount required by an individual varies with factors such as age, gender, and amount of physical activity. Riboflavin is found in many foods, such as eggs, nuts, grains, dairy products, organ meats, and dark green vegetables. Overall, riboflavin content in the body can be estimated by measuring the activity of glutathione reductase (a riboflavin-containing enzyme) in red blood cells. No one has been known to ever die of riboflavin deficiency, but it can occur as a consequence of malnourishment, intake of certain medication, chronic diarrhea, or alcoholism. The first symptoms of riboflavin deficiency are often light sensitivity, blurred vision, and bloodshot eyes. Other symptoms are skin and mucous membranes lesions. Because riboflavin is water-soluble and easily excreted , toxicity resulting from excess intake is not considered a health problem.
Riboflavin is important biochemically because it is vital for proper utilization of carbohydrates, fats, and proteins as energy sources. It is a component
of two coenzymes, flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN). (Coenzymes are molecules that must be added to certain polypeptides to make them functional enzymes.) In general, FAD and FMN, when tightly bound to specific enzymes, easily lose or gain one or two electrons, or hydrogen atoms, and so drive oxidation /reduction reactions. In the 1930s, Warburg and Christian studied "the old yellow enzyme," a riboflavin-requiring enzyme, and laid the groundwork for our current understanding of cyclic oxidation-reduction reactions in electron transport systems vital to cell respiration. In addition to their role in electron transport chains, FAD- and FMN-requiring enzymes catalyze reactions that are part of a wide array of metabolic pathways.
SEE ALSO Coenzyme .
Sharron W. Smith
Bibliography
Garrett, Reginald H., and Grisham, Charles M. (2002). Principles of Biochemistry: With a Human Focus. Fort Worth, TX: Harcourt College Publishers.
Robinson, Corinne H.; Lawler, Marilyn R.; Chenoweth, Wanda L.; et al. (1986). Normal and Therapeutic Nutrition, 17th edition. New York: Macmillan.
Internet Resources
Nutrition.org. Information available from http://www.nutrition.org .
Comment about this article, ask questions, or add new information about this topic: