Radiation
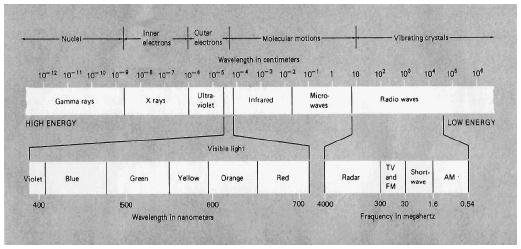
Radiation takes many forms, including both electromagnetic waves and sub-nuclear particles. The electromagnetic spectrum consists of light waves ranging in length from very short (10 −16 meters, or 3.937 × 10 −15 inches) to very long (10 8 meters, or 621,400 miles). The product of the velocity of electromagnetic waves and their wavelength is a constant equal to the velocity of light, 3 × 10 8 meters per second (m/s); therefore, as the length of waves increases, the frequency decreases. Thus, if the waves were 1 meter(3.3 feet) long, the frequency would be 3 × 10 8 hertz (Hz) or 300,000,000/s (meaning 300,000,000 light waves would pass by each second). The electromagnetic spectrum consists of light waves ranging in length from very short γ (gamma) rays through x rays, ultraviolet (UV) rays, the spectrum of visible light, infrared (IR) rays, and microwaves, to very long radio and television waves.
Forms of Radiation
The shortest electromagnetic waves are classified as γ rays. One of the forms of energy emanating from natural sources of radioactivity here on Earth and also from energy sources in space, γ rays can be thought of as very short x rays. Discovered by the German physicist Wilhelm Conrad Röntgen in 1895, the remarkable penetrating effect of rays and x rays results from their very short wavelength (from about 10 −12 to 10 −8 meters, or 3.28 × 10 −11 to 3.28 × 10 −7 feet). The waves are so small that they can pass through many substances with little interaction. X rays pass through skin and organs with little effect but are diffracted somewhat when they pass through denser materials such as bone; the resulting pattern enables technicians to make xray images of bones and of the contents of packages in airport scanners.
The energy of electromagnetic radiation is directly proportional to the frequency. Since both x rays and γ rays have very high frequencies, they carry large amounts of energy, and high intensities of x rays and γ rays can damage many materials (including living tissue). The rays may be focused by special lenses and used to kill cancer cells or organisms that might cause disease or hasten spoilage in food.
Bonds between atoms in chemical compounds vibrate at characteristic frequencies. Some molecules possess bonds capable of absorbing electromagnetic
energy, causing the bonds to bend, stretch, or vibrate and sometimes break. Certain bonds in particular (e.g., that between carbon and oxygen atoms) capture energy at specific frequencies of IR radiation, allowing technicians to use instruments called spectrophotometers to detect the presence of these bonds in chemical compounds. UV, visible, and IR spectroscopy are tools that permit chemists to readily identify and characterize small amounts of chemical substances.
Most animals, including humans, have visual receptors that detect light in the visible spectrum ranging from 400 nanometers (15.75 × 10 −6 for blue light to 700(27.56 × 10 −6 inches) for red light. (A nanometer equals 10 −9 meters.) Just below the visible spectrum lies UV light, ranging in wavelength from about 10 to 400 nanometers (3.937 × 10 −7 to 157.5 × 10 −7 inches). UV light is more energetic than visible light; UV radiation in the sunlight can damage molecules in the skin and is the cause of sunburn. Green plants carry out photosynthesis by using chlorophyll molecules that readily capture light energy in the visible spectrum.
Microwave radiation consists of electromagnetic waves somewhat longer than infrared waves (from about 10 −3 meters, or 0.3937 inches, to 1 meter, or 39.37 inches, long) and having a lower frequency, ranging from about 1,000 to 300,000 megahertz (MHz). Waves in this range are readily absorbed by bonds in water molecules. Microwave ovens take advantage of the fact that foods usually contain large amounts of water, and dishes do not. The waves of IR or microwave radiation are usually too long to pass through the small holes in the doors of microwave ovens; thus, one can use a microwave oven to heat food without heating the dish or being harmed by the radiation. Magnetron tubes generate radiation that can be used for radar or for the microwave transmission of electronic signals.
An immense amount of radiation passes undetected through the environment. Our surroundings contain large amounts of radio waves, generally from one to thousands of meters long. X rays and rays also pass through us and the space around us with little effect. From time to time, fears have been raised concerning the electromagnetic radiation emanating from power lines, cathode ray terminals such as television sets and computer monitors, and the earphones of personal transistor radios or CD players, but there is little actual evidence of injury or illness from low intensity radiation. However, workers have been injured or killed by high intensity microwave radiation, and technicians working with radioactive materials must take special precautions.
Much radiation arrives on Earth from the Sun, and some of the energy of this radiation exists in the form of UV light. UV light waves can damage skin and would be much more hazardous were it not for the layer of ozone that exists in Earth's upper atmosphere. In a process known as the Chapman cycle, UV radiation splits oxygen molecules (O 2 ) in the stratosphere to form free oxygen atoms (O). Some of these atoms combine with oxygen molecules to form ozone molecules (O 3 ). The O 3 molecules are especially sensitive to UV radiation; the absorption of UV photons converts the ozone back into oxygen atoms and oxygen molecules:
UV
O
2
→ 2 O
O + O
2
→ O
3
UV
O
3
→ O
2
+ O (1)
In recent years the amount of O 3 in the stratosphere over the South Pole has decreased periodically, resulting in an ozone "hole" in the atmosphere. The decrease is most pronounced during the summer months of the Southern Hemisphere. If the amount of ozone continues to decrease, more UV light will reach the surface of Earth, probably causing some skin damage and increasing the incidence of cancer. Chlorine atoms react with and destroy ozone:
C1 + O 3 → C1O + O 2 (2)
Increasing atmospheric amounts of chlorine atoms or free radicals probably result in the destruction of ozone; the source of the chlorine atoms is thought to be synthetic substances known as chlorofluorocarbons (CFCs) . Some CFCs may be released from air-conditioning equipment or aerosol spray cans, and some may result from the production of plastic foams. Several international agreements, including the Montréal Protocol of 1987 and the Copenhagen amendment of 1992, have been established to limit the production of CFCs.
Radioactivity
Although many forms of electromagnetic radiation exist, special consideration is often given to radiation from unstable (radioactive) atomic nuclei. This radiation is usually one of three types, α - and β -particles or γ rays, but some nuclear reactions may also result in the emission of neutrons. α -particles are relatively large and highly charged particles identical with the nuclei of helium atoms. Each has a mass of four atomic mass units (AMU) and a charge of +2. Radioactive ores containing sources of α -particles often produce helium gas as a result of the capture of electrons by the
α -particles. β -particles are high-speed electrons, having a mass of about 1/1,800 amu and a charge of −1. γ rays are similar to x rays.
X rays, γ rays, and neutron beams are considered ionizing radiation. Ionizing radiation may break molecules into pieces, creating ionic free radicals that can be very damaging to tissue. Contaminated food or dust containing radionuclides that emit α - and β -particles may be very dangerous if the sources of radiation are ingested. Strontium-90 ( 90 Sr) present in fallout from nuclear weapons testing may be absorbed from soil, incorporated in plant tissues, eaten by cows, and eventually find its way into milk. Strontium is chemically similar to calcium. The 90 Sr may then be absorbed from the digestive tract and deposited in bone, where α -particles released by radionuclide decay damage the blood-producing reactions in bone marrow.
Several methods are used to detect radiation; the earliest of these, also discovered by Röntgen, is exposure of photographic film. Since x rays can pass through solid materials, they expose photographic film sealed in lightproof envelopes. Workers in industrial settings today often wear film badges that contain a sheet of photographic film inside a plastic container fitted with aluminum and lead shields. In order to determine the amount and type of radiation exposure, the badges are collected periodically and the film developed. Darkening of the film indicates exposure. Workers may also wear dosimeters, pencil-like tubes that are examined daily for exposure. For large-scale operations or as survey monitors at factory gates, Geiger-Mueller counters are utilized. These devices detect radiation by using a tube consisting of a metal can containing a charged wire. The tube is sealed with a thin plastic or mica window. Radiation penetrating the can or window ionizes molecules of gas inside the tube, and the ions allow an electrical discharge, which can be detected and registered by an electrical circuit. Civil defense and military personnel often carry Geiger counters to survey large areas for contamination by radioactivity.
Uses of Radiation
Radiation is a versatile tool for the diagnosis and treatment of disease, as well as a means of industrial testing and treating foods to avoid spoilage. Many common metabolic substances can be labeled by replacing atoms such as carbon or hydrogen with radioactive atoms such as 14 C or 3 H. The resulting molecules are absorbed by the body and react in the same way as nonradioactive molecules, but the decay of the radioisotope releases tiny amounts of radioactivity that can be detected with sensitive instruments. Some compounds are absorbed more rapidly by diseased tissue (a cancerous organ, for example, might rapidly absorb glucose from blood), and the use of substances such as radioactive iodine can help diagnose tumors of the thyroid gland without invasive surgery. Some diseases such as cancer can be treated by administering a preparation containing radionuclides within molecules that are taken up by an organ and release their radioactivity within the diseased tissue.
Cells that are growing and dividing rapidly are the most sensitive to radiation. In the human body, these include gonadal tissue, hair follicles, the immune system, bone marrow, intestinal epithelium , and cancer cells. Cancer may be treated by external beam radiation, using γ -type radiation to deliver energy to abnormal cells, in the hope of killing them. Normal tissues are protected by lead shielding, and also by rotating the radiation source, passing the beam of rays through a larger range of tissue, and avoiding the intense irradiation of nontumorous tissues. In some cases, malignant cells can be treated with brachytherapy, the implantation of tiny metallic seeds containing radioisotopes that emit small amounts of radiation, killing the cancer from the inside. Radioisotopes decay at a known rate; often a nuclide that decays rapidly may be chosen, allowing the patient to be radiation-free upon discharge.
Gamma radiation is widely used in manufacturing to make images of welds in pipes. A recent application of radiation in the food industry involves the use of radiation (usually either γ rays or high-energy beams of electrons) to irradiate food, killing organisms that cause spoilage. The irradiation of food presents several advantages: foodstuffs may last weeks longer with little refrigeration; the use of dangerous chemical preservatives can be avoided; and foods may be prepared, wrapped, and preserved with less contact by human workers, lessening the chances of spreading disease-producing organisms. In recent years, especially virulent and damaging strains of Escherichia coli have caused outbreaks of illness among persons who consumed contaminated hamburger, and some cases of salmonella poisoning have been associated with the consumption of poultry products. Food irradiation may help to make such foods much safer.
Our environment contains many sources of radiation, such as cosmic rays that constantly bombard Earth. The atmosphere filters out some cosmic rays, so exposure is greater at higher altitudes than at sea level. Radiation sources also include smoke detectors, luminous watch dials, television and computer monitors, and medical x rays. We are exposed daily to electromagnetic radiation in the form of radio waves as well as α - and β -particles and γ rays emanating from radioactive carbon, hydrogen, and potassium, which are part of all living things. A small amount of radiation is probably harmless and may, in fact, be helpful. Radon in our homes is a potential cause of cancer. Although radon is a colorless, odorless gas, it decays to more chemically reactive and radioactive products that may bind in lung tissue. Like many dangerous gases, radon is much more hazardous in the presence of particulate matter such as the tiny particles present in cigarette smoke.
SEE ALSO Photosynthesis ; Radioactivity ; RÖntgen, Wilhelm .
Dan M. Sullivan
Bibliography
Christie, Maureen (2001). Ozone Layer. A Philosophy of Science Perspective. New York: Cambridge University Press.
Dessler, Andrew (2000). The Chemistry and Physics of Stratospheric Ozone. San Diego: Academic Press.
Miller, E. Willard, and Miller, Ruby M. (1990). Environmental Hazards: Radioactive Materials and Wastes: A Reference Handbook. Santa Barbara, CA: ABC-CLIO.
Molins, R. A., ed. (2001). Food Irradiation: Principles and Application. New York: Wiley.
Stanitski, Conrad L.; Eubanks, Lucy P.; Middlecamp, Catherine H.; and Pienta, Norman J. (2003). Chemistry in Context: Applying Chemistry to Society, 4th edition. Boston: McGraw-Hill.
Comment about this article, ask questions, or add new information about this topic: