Quantum Chemistry
Quantum chemistry is the application of quantum mechanical principles and equations to the study of molecules. In order to understand matter at its most fundamental level, we must use quantum mechanical models and methods. There are two aspects of quantum mechanics that make it different from previous models of matter. The first is the concept of wave-particle duality; that is, the notion that we need to think of very small objects (such as electrons) as having characteristics of both particles and waves. Second, quantum mechanical models correctly predict that the energy of atoms and molecules is always quantized, meaning that they may have only specific amounts of energy. Quantum chemical theories allow us to explain the structure of the periodic table, and quantum chemical calculations allow us to accurately predict the structures of molecules and the spectroscopic behavior of atoms and molecules.
Background: Wave-Particle Duality for Light
Quantum mechanical ideas began with studies of the physics of light. By the late nineteenth century, virtually all scientists believed that light behaved as a wave. Although some earlier scientists, such as Isaac Newton in the seventeenth century, had thought of light as consisting of particles, the early nineteenth-century experiments of Thomas Young and Augustin Fresnel demonstrated that light has wavelike properties. In these experiments, light was passed through a pair of slits in a screen, and produced alternating light and dark regions (interference patterns) on a second screen. This phenomenon, known as diffraction, cannot be explained using a particle model for light. In the late nineteenth century, James Clerk Maxwell derived a set of equations based on the wave model for light, which beautifully explained most experimental results.
Despite this apparent certainty that light was a wave, Max Planck and Albert Einstein, at the beginning of the twentieth century, showed that some experiments required the use of a particle model for light, rather than a wave model. Since both models were necessary for an accurate description of all of the properties of light, scientists today use mathematical equations appropriate to both waves and particles in describing the properties of light.
Waves and particles are fundamentally different: a particle exists at a particular point in space, whereas a wave continues on for (sometimes) a great distance. It defies intuition to think that both of these models might describe the same thing. Nevertheless, an accurate description of light requires the use of both wave and particle ideas.
The Wave Nature of Matter
The success of wave-particle duality in describing the properties of light paved the way for using that same idea in describing matter. Experiments in the early twentieth century showed that the energy in atoms is quantized ✶ ; that is, a given atom can have only specific amounts of energy. For hydrogen, the simplest of the atoms, an accurate formula for the possible energies had been experimentally determined but was unexplainable using any particle model for the atom. The best picture that the particle model could give, consistent with experiments on atoms, put the electron in a sort of "orbit" around the nucleus. Unfortunately, the particle model predicts that the electron should collide with the nucleus, releasing energy in the process. Obviously there was a need for a different model for the electron.
✶ One example of quantization would be to think of your distance from the ground when standing on a ladder—your distance from the ground can only change one rung at a time.
In 1924 Louis de Broglie presented a theory for the hydrogen atom that modeled the electron as a wave. Calculations made for this model give the quantization of energy that is experimentally observed in this atom. De Broglie also postulated a general formula for obtaining the wavelength of a moving object. His formula, which is analogous to that used for light, states that the wavelength of a moving object is inversely proportional to its momentum (mass times velocity). When one uses de Broglie's formula to determine wavelengths of macroscopic objects, one discovers that the wavelengths of even the smallest objects visible to the naked eye are too small for the wavelike characteristics of these objects to be significant in any real situation. For the electron, however, the wavelength is large enough to be measurable. Diffraction experiments have been performed using electrons, demonstrating conclusively that they have wave properties.
It is contrary to our intuition that electrons might behave as waves. The repercussions of this notion are that the electron does not have a definite size, but is spread out over a region in space. We are more comfortable with the thought of the electron being a microscopic particle, moving around in an orbit near the nucleus of an atom. As with light, however, we do not abandon the particle model for electrons; rather, we employ mathematical equations arising from both particle and wave models. For quantum chemical calculations, the wave model turns out to be more useful.
The Heisenberg Uncertainty Principle
One consequence of the wave nature of matter is that the position and momentum of small objects are not well known, as they would be for a particle model. In some circumstances, a wave may be confined to a very narrow region in space; however, there is still some uncertainty as to its position. Additionally, the value of the momentum of a quantum object is often not known precisely. In 1927 Werner Heisenberg showed that the product of the uncertainty in position and the uncertainty in momentum is greater than or equal to a certain constant (Planck's constant divided by 4 π ). This constant is very small; accordingly, quantum mechanical uncertainty in position and momentum of objects that are large enough to see is not noticed experimentally. For electrons, however, quantum mechanical uncertainties in position and momentum are important considerations in interpreting both theoretical models and experimental results. The relationship between the uncertainties in position and momentum is known as the Heisenberg Uncertainty Principle. It tells us that the more we know about the position of a small object, such as an electron, the less we know about its momentum (and vice versa).
Calculating the Wavefunction
For a scientist, knowing that matter behaves as a wave is useful only if one knows something about that wave. The wavefunction is a mathematical function describing the wave. For example, y ( x ) = A sin( kx ) might be the wave-function for a one-dimensional wave, which exists along the x -axis. Matter waves are three-dimensional; the relevant wavefunction depends on the x, y, and z coordinates of the system being studied (and sometimes on time as well). We conventionally label the wavefunction for a three-dimensional object as ψ ( x, y, z ). In 1926 Erwin Schrödinger introduced a mathematical equation whereby, if one knows the potential energy acting on an object, one can calculate the wavefunction for that object. Heisenberg had already introduced a mathematical formalism for performing quantum mechanics calculations, without explicitly including the concept of waves. It was later shown that, although the approaches of Schrödinger and Heisenberg looked very different, they made exactly the same predictions. In practice, the Schrödinger formalism is more useful for explaining the problem being studied, and the Heisenberg methodology allows for more straightforward computation. Accordingly, a mixture of the two approaches is typically used in modern quantum chemistry. Once we know the wavefunction of the atom or molecule under study, we can calculate the properties of that atom or molecule.
Quantum Mechanics of Atoms
An exact solution for Schrödinger's wave equation can be obtained for the hydrogen atom; however, for larger atoms and molecules (which contain more than one electron), Schrödinger's equation can be solved only approximately. Although this may sound so restrictive as to make the equation useless, there are well-established approaches that allow for practical and accurate calculations on atoms and molecules. This is done by making some assumptions about larger systems based upon the hydrogen atom, as explained below.
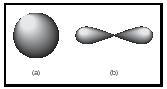
When the Schrödinger equation is solved for the hydrogen atom, the resulting wavefunctions for the various possible energies that the atom can have are used to determine atomic orbitals . An orbital is a region in space where an electron is most likely to be found. For example, the lowest-energy wavefunction for a hydrogen atom is the so-called 1s orbital (see Figure 1), which is a spherical region in space surrounding the nucleus. For some higher-energy states, the orbitals are not necessarily spherical in shape (e.g., the 2p orbital pictured in Figure 1).
For atoms larger than hydrogen, one assumes that the orbitals occupied by the electrons have the same shape as the hydrogen orbitals but are differing in size and energy. The energies corresponding to these orbitals may be found by solving an approximate version of Schrödinger's equation. These atomic orbitals, in turn, may be used as the building blocks to the electronic behavior in molecules, as we shall see below. As it happens, two electrons may share an atomic orbital; we say that these electrons are paired. Chemists have developed a system of rules for determining which orbitals are occupied in which atoms; calculations can then be done to determine the energies of the electrons in the atoms.
For atoms larger than hydrogen, one assumes that the orbitals occupied by the electrons have the same shape as the hydrogen orbitals but are differing in size and energy. The energies corresponding to these orbitals may be found by solving an approximate version of Schrödinger's equation. These atomic orbitals, in turn, may be used as the building blocks to the electronic behavior in molecules, as we shall see below. As it happens, two electrons may share an atomic orbital; we say that these electrons are paired. Chemists have developed a system of rules for determining which orbitals are occupied in which atoms; calculations can then be done to determine the energies of the electrons in the atoms.
Quantum Mechanics of Molecules
Molecules are held together by covalent bonds . The simplest definition of a covalent bond is a shared pair of electrons. There are two basic approaches to modeling covalent bonds in molecules: the valence bond model and the molecular orbital model. In the valence bond model, we think of atomic orbitals on each of two atoms combining to form a bond orbital, with one electron from each atom occupying this orbital. Both the bond orbital and the electron pair now "belong" to both of the atoms. This sharing of electrons brings about a lowering in the energy, which makes the formation of molecules from atoms an energetically favorable process. The valence bond model gives the simplest quantum mechanical picture of chemical bonding, but it is not the best method for accurate calculations on molecules containing more than two atoms.
Molecular orbital theory differs from valence bond theory in that it does not require the electrons involved in a bond to be localized between two of the atoms in a molecule. Instead, the electron occupies a molecular orbital, which may be spread out over the entire molecule. As in the valence bond approach, the molecular orbital is formed by adding up contributions from the atomic orbitals on the atoms that make up the molecule. This approach, which does not explicitly model bonds as existing between two atoms, is somewhat less appealing to the intuition than the valence bond approach. However, molecular orbital calculations typically yield better predictions of molecular structure and properties than valence bond methods. Accordingly, most commercially available quantum chemistry software packages rely on molecular orbital methods to perform calculations.
SCHRÖDINGER'S WAVE EQUATION
Schrödinger's Wave equation may be written (in abbreviated form) as:
Ê K ψ ( x, y, z ) + Ê P ψ ( x, y, z ) = Êψ ( x, y, z )
The first term, Ê K ψ ( x, y, z ), represents the kinetic energy of the system being studied. The second term, Ê P ψ ( x, y, z ), represents the potential energy of the system. E and ψ( x, y, z ) are the total energy of the system and wavefunction describing the system, respectively. Once the wavefunction is determined, virtually any property of the molecule may be calculated.
A lot of the modern research in quantum chemistry is focused on improving the valence bond and molecular orbital methods for calculating molecular properties. Different underlying approximations and different orbital functions are tried, and the results are compared with previous calculations and with experimental data to determine which methods give the best results. It is often the case that the best choice of quantum chemical method depends on the particular molecule or molecular property being studied.
SEE ALSO Atomic Structure ; Computational Chemistry ; Molecular Structure ; Theoretical Chemistry .
Wayne B. Bosma
Bibliography
Gribbin, John (1984). In Search of Schrödinger's Cat: Quantum Physics and Reality. New York: Bantam Books.
Jammer, Max (1966). The Conceptual Development of Quantum Mechanics. New York: McGraw-Hill.
Whitaker, Andrew (1996). Einstein, Bohr, and the Quantum Dilemma. New York: Cambridge University Press.