Nucleotides
Nucelotides are the repeating building blocks of nucleic acids (which are polynucleotides or polymers of nucleotides). A nucleotide is made up of a heterocyclic base (a purine or pyrimidine), a cyclic sugar unit (ribose or deoxyribose), and a phosphate group. A nucleotide is either a ribonucleotide, the repeating unit in ribonucleic acid ( RNA ), or a deoxyribonucleotide, the repeating unit in deoxyribonucleic acid ( DNA ). Table 1 below lists the names of purine and pyrimidine bases, the nucleosides (base + ribose), the corresponding 5′-nucleotides (base + ribose + phosphate), and the abbreviations.
Nucleotides are sometimes abbreviated as 5′-NMP, in which N can stand for any of the bases.
Figure 1 illustrates two nucleotides, in which the phosphate is attached to the (ribose) sugar at either the 5′-carbon (5′-AMP) or the 3′-carbon (3′-TMP). The numbering systems for both purine ( guanine shown here) and pyrimidine ( thymine shown here) compounds are given, in addition to that for the sugar (ribose or deoxyribose). Note that the linkage between the

| Base | Nucleoside | Nucleotide | Abbreviation |
| (These nucleotides are generally abbreviated, 5′-NMP, where N can contain any of the bases.) | |||
| Adenine | Adenosine | Adenosine-5′-(mono)phosphate | 5′-AMP |
| Guanine | Guanosine | Guanosine-5′-(mono)phosphate | 5′-GMP |
| Cytosine | Cytidine | Cytidine-5′-(mono)phosphate | 5′-CMP |
| Thymine | Thymidine | Thymidine-5′-(mono)phosphate | 5′-TMP |
| Uracil | Uridine | Uridine-5′-(mono)phosphate | 5′-UMP |
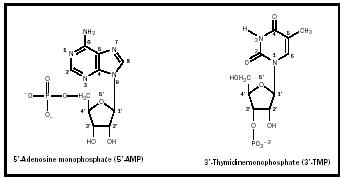
(deoxy)ribose unit and the base, a β -glycosidic bond, has a different connectivity according to whether the link is to a purine or a pyrimidine. Although the linkage involves a C1′ carbon in both cases, the β -glycosidic bond in the case of a purine nucleotide is a link to the N-9 of the purine base , but to the N-1 of the pyrimidine base .
Nucleotides are also found in which two or three phosphate groups are linked together, the chain of phosphate groups bonded to the sugar's 5′-position. In these cases, they are nucleoside diphosphates (5′-NDP) and nucleoside triphosphates (5′-NTP).
The bases have very limited solubilities in water, whereas the nucleosides and nucleotides have greater solubilities, due to the presence of polar sugars, or of both sugars and charged phosphate groups, respectively.
The nucleoside triphosphates are of special interest for at least two reasons. First, they are the actual precursor molecules used in the biosynthesis of nucleic acids. Second, ATP is a high-energy molecule, produced primarily in mitochondria during oxidative phosphorylation . It stores energy, which is released (dG° = −7.3 kcal/mol) during the hydrolysis of ATP to ADP and phosphate (Pi), as shown, and then utilized to power cell reactions.
ATP + H 2 O → ADP + Pi
SEE ALSO Deoxyribonucleic Acid (DNA) ; Restriction Enzymes .
William M. Scovell
Bibliography
Berg, Jeremy M.; Tymoczko, John L.; and Stryer, Lubert (2002). Biochemistry, 5th edition. New York: W. H. Freeman.
Lehninger, Albert L. (2000). Lehninger Principles of Biochemistry, 3rd edition, ed. David L. Nelson and Michael M. Cox. New York: Worth Publishers.
Comment about this article, ask questions, or add new information about this topic: