Molecular Geometry
Molecules, from simple diatomic ones to macromolecules consisting of hundreds of atoms or more, come in many shapes and sizes. The term "molecular geometry" is used to describe the shape of a molecule or polyatomic ion as it would appear to the eye (if we could actually see one). For this discussion, the terms "molecule" and "molecular geometry" pertain to polyatomic ions as well as molecules.
Molecular Orbitals
When two or more atoms approach each other closely enough, pairs of valence shell electrons frequently fall under the influence of two, and sometimes more, nuclei. Electrons move to occupy new regions of space (new orbitals—molecular orbitals) that allow them to "see" the nuclear charge of multiple nuclei. When this activity results in a lower overall energy for all involved atoms, the atoms remain attached and a molecule has been formed. In such cases, we refer to the interatomic attractions holding the atoms together as covalent bonds. These molecular orbitals may be classified according to strict mathematical (probabilistic) determinations of atomic behaviors. For this discussion, the two most important classifications of this kind are sigma ( σ ) and pi ( π ). Though we may be oversimplifying a highly complex mathematics, it may help one to visualize sigma molecular orbitals as those that build up electron density along the (internuclear) axis connecting bonded nuclei, and pi molecular orbitals as those that build up electron density above and below the internuclear axis.
Bonding Theories
This discussion will examine two approaches chemists have used to explain bonding and the formation of molecules, the molecular orbital (MO) theory and the valence bond (VB) theory. At their simplest levels, both approaches ignore nonvalence shell electrons, treating them as occupants of molecular orbitals so similar to the original (premolecular formation) atomic orbitals that they are localized around the original nuclei and do not participate in bonding. The two approaches diverge mainly with respect to how they treat the electrons that are extensively influenced by two or more nuclei. Though the approaches differ, they must ultimately converge because they describe the same physical reality: the same nuclei, the same electrons.
Molecular orbital theory. In MO theory, there are three types of molecular orbitals that electrons may occupy.
1. Nonbonding molecular orbitals. Nonbonding molecular orbitals closely resemble atomic orbitals localized around a single nucleus. They are called nonbonding because their occupation by electrons confers no net advantage toward keeping the atoms together.
2. Bonding molecular orbitals. Bonding molecular orbitals correspond to regions where electron density builds up between two, sometimes more, nuclei. When these orbitals are occupied by electrons, the electrons "see" more positive nuclear charge than they would if the atoms had not come together. In addition, with increased electron density in the spaces between the nuclei, nucleus-nucleus repulsions are minimized. Bonding orbitals allow for increased electron-nucleus attraction and decreased nucleus-nucleus repulsion, therefore electrons in such orbitals tend to draw atoms together and bond them to each other.
3. Antibonding molecular orbitals. One antibonding molecular orbital is formed for each bonding molecular orbital that is formed. Antibonding orbitals tend to localize electrons outside the regions between nuclei, resulting in significant nucleus-nucleus repulsion—with little, if any, improvement in electron-nucleus attraction. Electrons in antibonding orbitals work against the formation of bonds, which is why they are called antibonding.
According to MO theory, atoms remain close to one another (forming molecules) when there are more electrons occupying lower energy sigma and/or pi bonding orbitals than occupying higher energy antibonding orbitals; such atoms have a lower overall energy than if they had not come together. However, when the number of bonding electrons is matched by the number of antibonding electrons, there is actually a dis advantage to having the atoms stay together, therefore no molecule forms.
Valence bond theory. Valence bond (VB) theory assumes that atoms form covalent bonds as they share pairs of electrons via overlapping valence shell orbitals. A single covalent bond forms when two atoms share a pair of electrons via the sigma overlap of two atomic orbitals—a valence orbital from each atom. A double bond forms when two atoms share two pairs of electrons, one pair via a sigma overlap of two atomic orbitals and one via a pi overlap. A triple bond forms by three sets of orbital overlap, one of the sigma type and two of the pi type, accompanied by the sharing of three pairs of electrons via those overlaps. (When a pair of valence shell electrons is localized at only one atom, that is, when the pair is not shared between atoms, it is called a lone or nonbonding pair.)
Let us apply this greatly simplified picture of VB theory to three diatomic molecules: H 2 , F 2 , and HF. VB theory says that an H 2 molecule forms when a 1 s orbital containing an electron that belongs to one atom overlaps a 1 s orbital with an electron of opposite spin belonging to the other, creating a sigma molecular orbital containing two electrons. The two nuclei share the pair of electrons and draw together, giving both electrons access to the positive charge of both nuclei. Diatomic fluorine, F 2 , forms similarly, via the sigma overlap of singly occupied 2 p orbitals. The HF molecule results from the sharing of a pair of electrons whereby an electron in a hydrogen 1 s orbital experiences sigma overlap with an electron in a fluorine 2 p orbital.
Molecular Geometries
This VB approach allows us to return to the focus of our discussion. The geometry of a molecule or polyatomic ion is determined by the positions of individual atoms and their positions relative to one another. It can get very complicated. However, let us start with some simple examples and your imagination will help you to extend this discussion to more complicated ones. What happens when two atoms are bonded together in a diatomic molecule? The only possible geometry is a straight line. Hence, such a molecular geometry (or shape) is called "linear." When we have three bonded atoms (in a triatomic molecule), the three atoms may form either a straight line, creating a linear molecule, or a bent line (similar to the letter V), creating a "bent," "angular," "nonlinear," or "V-shaped" molecule. When four atoms bond together, they may form a straight or a zigzag line, a square or other two-dimensional shape in which all four atoms occupy the same flat plane, or they may take on one of several three-dimensional geometries (such as a pyramid, with one atom sitting atop a base formed by the other three atoms). With so many possibilities, it may come as a surprise that we can "predict" the shape of a molecule (or polyatomic ion) using some basic assumptions about electron-electron repulsions.
We start by recognizing that, ultimately, the shape of a molecule is the equilibrium geometry that gives us the lowest possible energy for the system. Such a geometry comes about as the electrons and nuclei settle into positions that minimize nucleus-nucleus and electron-electron repulsions, and maximize electron-nucleus attractions.
Modern computer programs allow us to perform complex mathematical calculations for multiatomic systems with high predictive accuracy. However, without doing all the mathematics, we may "predict" molecular geometries quite well using VB theory.
Valence shell electron pair repulsion approach. In the valence shell electron pair repulsion (VSEPR) approach to molecular geometry, we begin by seeing the valence shell of a bonded atom as a spherical surface. Repulsions among pairs of valence electrons force the pairs to locate on this surface as far from each other as possible. Based on such considerations, somewhat simplified herein, we determine where all the electron pairs on the spherical surface of the atom "settle down," and identify which of those pairs correspond to bonds. Once we know which pairs of electrons bond (or glue) atoms together, we can more easily picture the shape of the corresponding (simple) molecule.
However, in using VSEPR, we must realize that in a double or triple bond, the sigma and pi orbital overlaps, and the electrons contained therein, are located in the same basic region between the two atoms. Thus, the four electrons of a double bond or the six electrons of a triple bond are not independent of one another, but form coordinated "sets" of four or six electrons that try to get as far away from other sets of electrons as possible. In an atom's valence shell, a lone pair of electrons or, collectively, the two, four, or six electrons of a single, double, or triple bond each form a set of electrons. It is repulsions among sets of valence shell electrons that determine the geometry around an atom.
Consider the two molecules carbon dioxide (CO 2 ) and formaldehyde (H 2 CO). Their Lewis structures are and
and
In CO 2 , the double bonds group the carbon atom's eight valence electrons into two sets. The two sets get as far as possible from each other by residing on opposite sides of the carbon atom, creating a straight line extending from one set of electrons through the carbon nucleus to the other. With oxygen atoms bonded to these sets of electrons, the oxygen–carbon–oxygen axis is a straight line, making the molecular geometry a straight line. Carbon dioxide is a linear molecule.
In H 2 CO, the carbon atom's eight valence electrons are grouped into three sets, corresponding to the two single bonds and the one double bond. These sets minimize the repulsions among themselves by becoming as distant from one another as possible—each set pointing at a vertex of a triangle surrounding the carbon atom in the center. Attaching the oxygen and hydrogen atoms to their bonding electrons has them forming the triangle with the carbon remaining in the center; all four atoms are in the same plane. Formaldehyde has the geometry of a trigonal (or triangular) planar molecule, "planar" emphasizing that the carbon occupies the same plane as the three peripheral atoms.


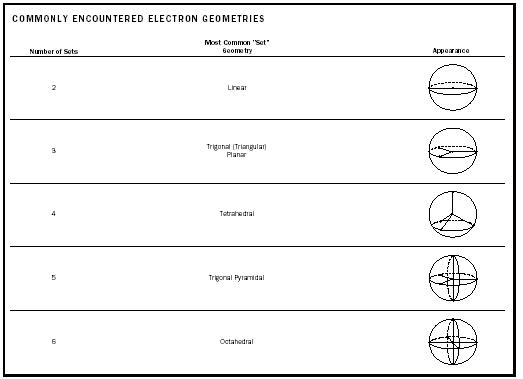
| COMMONLY ENCOUNTERED ELECTRON GEOMETRIES | ||
| Most Common "Set" | ||
| Number of Sets | Geometry | Appearance |
| 2 | Linear | |
| 3 | Trigonal (Triangular) Planar | |
| 4 | Tetrahedral | |
| 5 | Trigonal Pyramidal | |
| 6 | Octahedral | |
We may extend this approach to central atoms with four, five, six, or even more sets of valence shell electrons. The most common geometries found in small molecules appear in Table 1.
Until now, this article has focused on all the electrons in a central atom's valence shell, including sets not engaged in bonding. Though all such sets must be included in the conceptualization of the electron-electron repulsions, a molecule's geometry is determined solely by where its atoms are: A molecule's geometry is identified by what people would see if they could see atoms. In the carbon dioxide and formaldehyde examples, the molecules have the same overall geometries as the electron sets, because in both cases all sets are attached to peripheral atoms: Carbon dioxide is a linear molecule and formaldehyde is a trigonal (or triangular) planar one.
On the other hand, a water molecule (H 2 O)
has four sets of electrons around the O atom (two lone pairs and those making up two sigma bonds) that assume a tetrahedral arrangement, but the molecular geometry as determined by the positions of the three atoms is a bent, or V-shaped, molecule, with a H–O–H angle approaching the tetrahedral angle of 109.5°.
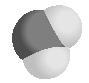
Similarly, a hydronium ion (H 3 O + )
has four sets of electrons around the central O atom (one lone pair and those making up three sigma bonds) in a tetrahedral arrangement, but the molecular geometry as determined by the four atoms is a trigonal (three-pointed base) pyramidal ion with the O atom "sitting" atop the three H atoms. The hydronium ion also has a H–O–H angle approaching the tetrahedral angle of 109.5°.

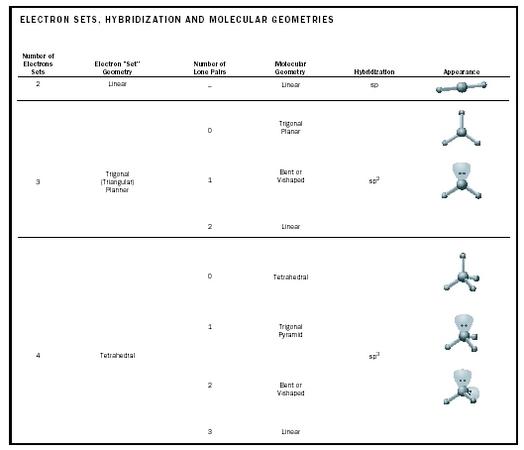
Table 2 outlines the most common molecular geometries for different combinations of lone pairs and up to four total sets of electrons that have assumed positions around a central atom, and the hybridizations (see below) required on the central atom.
Hybridization. Finally, what does valence bond theory say about the atomic orbitals demanded by VSEPR? For example, though the regions occupied by sets of electrons having a tetrahedral arrangement around a central atom make angles of 109.5° to one another, valence p -orbitals are at 90° angles.
To reduce the complex task of finding orbitals that "fit" VSEPR, we base their descriptions on mathematical combinations of "standard" atomic orbitals, a process called hybridization; the orbitals thus "formed" are hybrid orbitals. The number of hybrid orbitals is equal to the number of "standard" valence atomic orbitals used in the mathematics. For example, combining two p -orbitals with one s -orbital creates three unique and equivalent sp 2 ( s - p -two) hybrid orbitals pointing toward the vertices of a triangle surrounding the atom.
| ELECTRON SETS, HYBRIDIZATION AND MOLECULAR GEOMETRIES | |||||
| Number of | |||||
| Electrons | Electron "Set" | Number of | Molecular | ||
| Sets | Geometry | Lone Pairs | Geometry | Hybridization | Appearance |
| 2 | Linear | – | Linear | sp | |
| 0 | Trigonal Planar | ||||
| 3 | Trigonal (Triangular) Planner | 1 | Bent or V-shaped | sp 2 | |
| 2 | Linear | ||||
| 0 | Tetrahedral | ||||
| 1 | Trigonal Pyramid | ||||
| 4 | Tetrahedral | sp 3 | |||
| 2 | Bent or V-shaped | ||||
| 3 | Linear | ||||

Valence electron sets (lone pairs and electrons in sigma bonds) are "housed," at least in part, in hybrid orbitals. This means that an atom surrounded by three electron sets uses three hybrid orbitals, as in formaldehyde. There, the central carbon atom uses hybrid orbitals in forming the C–H single bonds and the sigma portion of the C=O double bond. The carbon's remaining unhybridized p -orbital overlaps a p -orbital on the oxygen, creating the pi bond that completes the carbon-oxygen double bond. The H–C–O and H–C–H angles are 120°, as is found among sp 2 hybridized orbitals in general. The hybridizations required for two, three, and four electron sets are given in Table 2, along with their corresponding electron geometries.
SEE ALSO Isomerism ; Lewis Structures ; Molecules ; Nuclear Magnetic Resonance .
Mark Freilich
Comment about this article, ask questions, or add new information about this topic: