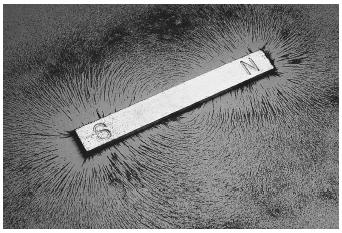
Magnetism
The magnetic properties of materials were recognized by the ancient Greeks, Romans, and Chinese, who were familiar with lodestone, an iron oxide mineral that attracts iron objects. Although the attractive or repulsive forces that act between magnetic materials are manifestations of magnetism familiar to everybody, the origin of magnetism lies in the atomic structure of matter. Despite the fact that magnetism can be explained only by the quantum theory developed at the beginning of the twentieth century, qualitative predictions of magnetic properties can be made within the context of classical physics. Magnetic forces originate in the motion of charged particles, such as electrons. The electrons "spin" around their axis and move in orbits around the nucleus of the atom to which they belong. Both motions generate tiny electric currents in closed loops that in turn create magnetic dipole fields, just as the current in a coil does. When placed in a magnetic field, the tiny magnetic dipole fields tend to align with the external field.
According to their behavior in inhomogeneous magnetic fields, materials can be classified into three main categories: diamagnetic , paramagnetic, and ferromagnetic. Paramagnetic materials are attracted into a magnetic field. The main cause of this effect is the presence in the material of atoms that have a net magnetic moment composed of electron spin and orbital contributions.
When placed in a magnetic field, the magnetic moments of the atoms, which are otherwise randomly oriented, tend to align with the field and thus enhance the field. Paramagnetism is temperature dependent because increased thermal motion at higher temperatures impedes the alignment of the magnetic moments with the field. Diamagnetic materials are slightly repelled by a magnetic field. This effect occurs for materials that contain atoms in which the spin and orbital contributions to the magnetic moment cancel out. In this case, the interaction between the material and a magnetic field is caused by the occurrence of currents induced by the magnetic field in the atoms. The dipole fields corresponding to these currents are directed opposite to the applied magnetic field and cause expulsion of the material from the field. Ferromagnetic materials contain atoms that have magnetic moments that are aligned even in the absence of an applied magnetic field because of mutual interactions, creating a sizable net magnetic moment for domains of the material. The magnetic moments of domains can be randomly oriented unless a magnetic field is applied to the material.
Iron, cobalt, nickel, and their alloys are examples of ferromagnetic materials. These three elements are transition metals , and their atoms or ions have unpaired electrons in d orbitals. Rare-earth ions also have unpaired electrons situated in f orbitals. A detailed investigation of the properties of molecules that contain such metal ions in a magnetic field can provide significant information about how their electrons are distributed in orbitals. Typically, d orbitals of isolated atoms are degenerate (Figure 1a). This situation changes when the metal ions are part of molecules in which they experience a nonspherically symmetric environment. Figures 1b and 1c show the splitting of d orbitals for a transition metal ion that has six unpaired electrons and is situated in an environment of six atoms in an octahedral arrangement. Depending on the size of the splitting (the lighter shading in Figure 1) and the interelectron repulsion, the metal ion may have four unpaired electrons (Figure 1b) or no unpaired electrons (Figure 1c). This difference in electron distribution leads to significant differences in the magnetic properties of the molecules that contain such ions, with
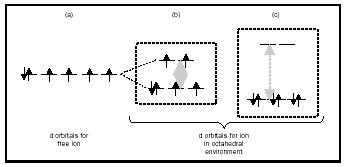
the former being paramagnetic and the latter being diamagnetic. When there are multiple metal sites in a molecule, the spins at different metal ions can be either ferro-(parallel) or antiferro-magnetically (antiparallel) aligned to each other. Clever use of the magnetic properties for metal ions and of the interactions between spins manifested in molecular systems enables scientists to design and synthesize molecular systems with interesting properties, such as molecular magnets.
Magnetic materials are widely used for building technological devices and scientific tools. Classical examples are electromagnets that are used in motors, clutches, and breaking systems. The electromagnet makes use of an iron core situated in a solenoid through which electric current is passed. This current creates a magnetic field at the center of the solenoid that orients the magnetic moments in the domains of the iron core, which in turn results in a significant enhancement of the magnetic field at the core of the solenoid. Electromagnets can also be used to record information on magnetic tape, which has a ferromagnetic surface.
Finally, although atomic nuclei have significantly smaller magnetic moments than electrons, the study of their interaction with magnetic fields has many important applications. They enable the scientists in the biological and medical fields to elucidate the structure of biologically relevant molecules such as proteins and to diagnose diseases using magnetic resonance imaging.
SEE ALSO Maxwell, James Clerk ; Physical Chemistry .
Catalina Achim
Bibliography
Kittel, C. (1996). Introduction to Solid State Physics , 5th edition. New York: Wiley.
Miessler, G. L., and Tarr, D. A. (1999). Inorganic Chemistry, 2nd edition. Upper Saddle River, NJ: Prentice Hall.