Liquids

A liquid is one state in which matter can exist. A liquid can take the shape of any container it is placed in (unlike a solid), but the volume of the liquid will always remain constant (unlike a gas).
Liquid-Particle Movement
On a molecular level the molecules of a liquid are arranged, or ordered somewhere between the order of a solid and the randomness of a gas. The particles comprising a solid occur in an ordered fashion, producing a characteristic three-dimensional configuration that is present throughout the entire structure. The forces between particles in a solid are strong, holding the particles in a rigid form. A solid is therefore noncompressible and cannot flow to take the shape of its container. Conversely, in a gas the particles have no regularity in their arrangement, have essentially unrestricted movement, and are widely separated. Because the forces between particles are small, the particles may move apart and fill the available space. The shape and volume of a gas may therefore be changed.
A molecule of a liquid experiences an environment similar to that of a solid—it is in close proximity to its neighbors and has similar packing density. In a liquid, however, particles have no long-range order (i.e., only on a localized basis). The intermolecular forces between particles of a liquid are stronger than the kinetic energies of the molecules, which are thus held close together. These forces, however, do not hold the molecules in a rigid structure. Subsequently the molecules can move with respect to each other, allowing a liquid to flow. A liquid is minimally compressible and much denser than a gas, and maintains a constant volume.
The particles comprising liquids can be molecules or atoms depending on the chemical nature of the substance. The general characteristics of a liquid are the same irrespective of its composition (molecules versus atoms) but hydrogen bonding can increase the attractive forces between molecules making a liquid flow less easily.
Decreasing the kinetic energy of particles of a liquid by cooling will eventually result in the change of state from liquid to solid. Similarly, increasing the kinetic energy by heating will result in the state change from liquid to gas. For example, at a pressure of one atmosphere, pure water changes from liquid to solid at 0°C (32°F) and from liquid to gas at 100°C (212°F).
Surface Tension
Surface tension is the appearance of a film over the top of a liquid, making the liquid behave as if it had a skin. It is because of surface tension that a small object like a pin or an insect can be supported on the surface of a liquid. This phenomenon is caused by the attraction between molecules of a liquid. In the bulk of a liquid any individual molecule is attracted equally in all directions by all adjacent molecules. At the surface of the liquid, however, there are no molecules attracting the surface molecules other than those in the liquid itself. This leads to a net attraction for the surface molecules into the liquid, in a direction parallel to the surface.
The most noticeable effect of surface tension is to reduce the surface of the liquid to the smallest possible size. Surface tension on water, for example,
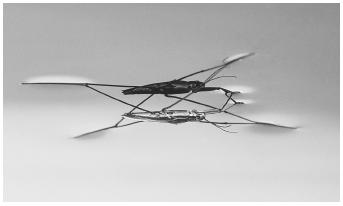
causes water droplets to form the familiar bead shape (particularly noticeable on a wax surface). This surface tension can be destroyed by the addition of a detergent, causing the water droplet to spread out to a thin film covering the surface of the container.
To expand the surface of a liquid, the forces pulling the particles inward must be overcome. Surface tension is a measure of the amount of energy required to increase the surface area of a liquid by a given amount. A liquid displaying hydrogen bonding will have a higher surface tension than one that does not. For example, at 20°C (68°F) water has a surface tension of 7.29 × 10 −2 joules per meter squared (the amount of energy that must be applied to water to increase the surface area by 1 meter squared). For mercury the surface tension is even higher (4.6 × 10 −1 joules per meter squared at 20°C); this is due to the stronger metallic bonds between the atoms of mercury.
Viscosity
Viscosity is a measure of how thick (viscous) and sticky a liquid is. Viscosity reduces the ability of a liquid to flow. Any liquid that can flow readily (such as water) will have a low viscosity. Liquids with a high viscosity (such as molasses and motor oil) will flow more slowly and with greater difficulty.
Viscosity can be measured by timing how long it takes for the liquid to flow through a capillary tube or how long it takes for a steel ball to fall through the liquid. At a molecular level viscosity is a function of the attractive forces of the molecules of the liquid and, to a lesser extent, the presence of structural components (such as long-chain molecules) that can become entangled (a form of steric hindrance). Temperature also greatly affects viscosity: as temperature increases, viscosity decreases. This is because higher levels of kinetic energy are more able to overcome the intermolecular attractive forces.
Liquid Crystals
The term "liquid crystal" describes an intermediate state between a solid and a liquid. On heating to a specific temperature, some solids become

cloudy liquids, then become clear when the temperature is raised even further. For such substances, the temperature range at which this intermediate state—called the liquid crystal state—exists is always the same. In 1888 Austrian botanist and chemist Frederich Reinitzer was the first to discover a substance (cholesterol benzoate) that exhibited this behavior. Cholesterol benzoate melts at 145°C (293°F) to form a cloudy, milklike liquid, but at 179°C (354°F) the liquid becomes clear.
Liquid crystals are composed of long, rodlike molecules that can be ordered in a number of ways. There are three groups of liquid crystals: cholesteric, nematic, and smectic. In the cholesteric form all of the molecules in one layer are aligned in the same manner, but adjacent layers have the molecules twisted with respect to each layer (commonly encountered in crystals of cholesterol from which the name originates). In the case of a nematic liquid crystal, the axes of the molecules are aligned but the ends of the molecules are not aligned or adjacent to each other. The smectic liquid crystal is characterized by the axes and the ends of the molecules being aligned. The ordering of the molecules is altered by changes in pressure, temperature, and electric and magnetic fields.
Glasses
Glasses are supercooled liquids that form a noncrystalline solid. The most frequently encountered glass is the supercooled form of liquid silicon dioxide—the glass used in windows, for example. When silicon dioxide is heated to 1,600°C (2,912°F), it forms a viscous liquid. Upon rapid cooling, silicon-oxygen bonds are formed before the atoms are able to arrange themselves in a regular pattern characteristic of a solid.
Glasses share a number of common characteristics regardless of their origins. All glasses are transparent or translucent, hard, brittle, and resistant to chemical attack. Glasses can be made from a range of acidic oxides including lead, boron, and phosphorus. When a glass is heated to its softening point, the material begins to crystallize and becomes more brittle and opaque. Toughening of glass can be brought about by rapid cooling during its production or by chemical treatment of the surface. To produce colored glass, metallic oxides or other compounds are added. For window glass other compounds can be added to make the characteristics more desirable; these include sodium carbonate, calcium oxide (lime), or calcium carbonate.
SEE ALSO Glass ; Molecular Structure ; Physical Chemistry .
Gordon Rutter
Bibliography
Brady, James E., and Holum, John R. (1993). Chemistry: The Study of Matter and Its Changes. New York: John Wiley and Sons.
Collings, Peter J., and Hird, Mike (1997). Introduction to Liquid Crystals: Chemistry and Physics. London: Taylor and Francis.
Hansen, Jean Pierre, and McDonald, Ian R. (1990). Theory of Simple Liquids. London: Academic Press.
Marcus, Yitzhak (1977). Introduction to Liquid State Chemistry. New York: John Wiley and Sons.
Comment about this article, ask questions, or add new information about this topic: