Chemical Equations
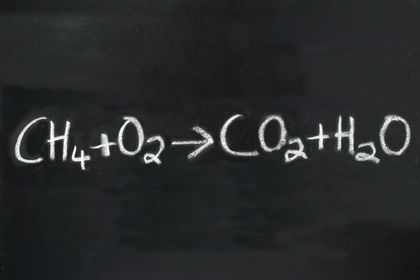
Chemical reactions convert reactants to products, whose properties differ from those of the reactants. Chemical equations are a compact and convenient way to represent chemical reactions. They have the general form
Reactant(s) → Product(s)
The arrow in the equation means "changes to" or "forms." The reaction of gaseous nitrogen with hydrogen to produce ammonia, NH 3 , is represented by the chemical equation
Although there are thousands of chemical reactions, a significant number of them, especially those that are not organic reactions, can be classified according to four general patterns: combination, decomposition, displacement, and exchange.
1. Combination. A combination reaction is one in which two or more substances (the reactants) are combined directly to form a single product (the product). An example is the reaction in which sodium (Na) combines with chlorine (Cl 2 ) to form sodium chloride, or table salt (NaCl).

2 Na + Cl 2 → 2 NaCl
The physical states of reactants and products are included where necessary. The symbols used are: ( s ) for solid, ( l ) for liquid, ( g ) for gas, and ( aq ) for aqueous (water) solutions. In the case of sodium chloride formation, the equation is modified accordingly.
2 Na ( s ) + Cl 2 ( g ) → 2 NaCl ( s )
2. Decomposition. A decomposition reaction can be considered to be the reverse of a combination reaction. In a decomposition reaction, one substance (the reactant) decomposes to form two or more products. For example, calcium carbonate (limestone) decomposes at high temperatures to calcium oxide (lime) and carbon dioxide. This reaction is used industrially to produce large quantities of lime.
3. Displacement. A displacement reaction (also called a single replacement reaction) occurs when an element reacts with a compound to form a new compound and release a different element. An example is the reaction that releases silicon (Si) from silicon dioxide (sand), SiO 2 , via its reaction with carbon. Carbon monoxide, CO, is the reaction's other product. When further purified, the silicon can be used in computer chips.
SiO 2 ( s ) + 2 C ( s ) → Si ( s ) + 2 CO ( g )
4. Exchange. During an exchange reaction, "partners" in compounds exchange their partners. One type of exchange reaction is called a neutralization reaction, the reaction between an acid and a base. The reaction of sodium hydroxide (lye), NaOH, with hydrochloric acid, HCl, to produce NaCl and water is such a reaction. In this case, Na + switches partners from OH − to Cl − , and H + from Cl − to OH − .
NaOH ( aq ) + HCl ( aq ) → NaCl ( aq ) + H 2 O ( l )
Organic chemical reactions, those in which carbon plays a predominant role, are very important in biochemical systems and industrial processes. These reactions can also be represented by balanced chemical equations, a few examples of which are given.
The fermentation of glucose to produce ethyl alcohol (ethanol)
The synthesis of acetylsalicylic acid (aspirin) from the reaction of salicylic acid with acetic anhydride
The formation of a triglyceride (a fat), such as the biochemical synthesis of tristearin via the reaction of stearic acid with glycerol:
Matter is conserved in chemical reactions: The total mass of the products equals the total mass of the reactants. Chemical equations reflect this conservation. It is why chemical equations must be balanced. Atoms have mass, and the numbers of each kind of atom on each side of the equation must be the same. Coefficients, the numbers to the left of the formulas, are used to balance equations. Many equations can be balanced directly by simply adjusting the coefficients, as illustrated in the equations given above. Other equations are more difficult to balance, such as that for the decomposition of nitroglycerine (an explosive)
4 C 3 H 5 (NO 3 ) 3 ( l ) → 12 CO 2 ( g ) + 10 H 2 O ( l ) + 6 N 2 ( g ) + O 2 ( g )
and this complicated reaction involving several reactants and products
4 CuSCN + 7 KIO 3 + 14 HCl → 4 CuSO 4 + 7 KCl + 4 HCN + 7 ICl + 5 H 2 O
Balanced chemical equations provide a significant amount of information. Consider the equation for photosynthesis, the natural process by which green plants form glucose, C 6 H 12 O 6 , and oxygen from the reaction of carbon dioxide with water.
This balanced equation and its coefficients can be interpreted as indicating that six carbon dioxide molecules and six water molecules react to form one molecule of glucose and six oxygen molecules, each containing two oxygen atoms. A coefficient multiplies the term following it. The "6 CO 2 " denotes six CO 2 molecules containing a total of six carbon atoms and twelve oxygen atoms.
Applying these concepts to the remainder of the balanced equation yields information that confirms that the equation is balanced—the atom counts for both sides of the equation are the same.

| Reactants | Products |
| Carbon atoms = 6 | Carbon atoms = 6 |
| Hydrogen atoms = 12 | Hydrogen atoms = 12 |
| Oxygen atoms = 12 + 6 = 18 | Oxygen atoms = 6 + 12 = 18 |
Coefficients also apply to a larger scale, in which the counting unit is the mole (there are 6.02 × 10 23 molecules per mole of a compound), rather than individual molecules. Thus, this balanced equation also represents the reaction of six moles of glucose with six moles of water to produce one mole of glucose and six moles of oxygen.
Oxidation-reduction (redox) reactions are an important, general kind of reaction, one involving the transfer of electrons. Oxidation is the loss of an electron or electrons from an element, ion, or compound. Reduction is the gain of an electron or electrons from an element, ion, or compound. The two processes occur simultaneously; electrons released during oxidation are gained in a reduction process. In every redox reaction, a reactant is oxidized (loses electrons) and a reactant is reduced (gains electrons). During a redox reaction there is a change in oxidation numbers—evidence of a redox reaction. An oxidation number compares the charge of an uncombined atom, one not in a compound, with its actual or relative charge when it is part of a compound. Oxidation numbers are zero, positive, or negative.
These guidelines are used to determine oxidation numbers.
- Atoms of pure elements, that is, atoms not combined with any other element, have an oxidation number of zero. For example, sodium in metallic sodium, Na; oxygen in molecular oxygen, O 2 ; and chlorine in molecular chlorine, Cl 2 , each have an oxidation number of 0.
- Monatomic ions have an oxidation number equal to the charge of the ion. Thus, a sodium ion, Na + , has an oxidation number of +1; that of chlorine in a chloride ion, Cl − , is −1.
- Generally, hydrogen atoms in compounds have an oxidation number of +1; oxygen atoms in compounds are typically −2.
- The sum of oxidation numbers in a neutral compound is zero. Water, H 2 O, is an example. Hydrogen: 2 H × (+1/H) = +2; oxygen: 1 O × (−2/O) = −2; (+2) + (−2) = 0
- The sum of oxidation numbers of the atoms in a polyatomic ion equals the charge on the ion. For example, the sulfate ion, SO 4 −2 , a polyatomic ion, has a net charge of −2. Each oxygen in a sulfate ion has an oxidation number of −2, and four oxygens add up to −8. For the sulfate ion to have a net −2 charge, sulfur must have a +6 oxidation number: −2 = 4(−2) +6.
Oxidation numbers and their changes can be used to identify the reaction of sodium with chlorine to form NaCl as a redox reaction.
2 Na + Cl 2 → 2 NaCl

| Reactant | Products |
| Oxidation number | Oxidation number |
| Na = 0 | Na + = +1 |
| Cl = 0 | Cl − = −1 |
During this reaction, reactant sodium atoms (oxidation number 0) are converted to sodium ions (oxidation number +1); reactant chlorine atoms (oxidation number 0) are transformed to chloride ions (oxidation number −1). Because there is a change in the oxidation numbers of the reactants during the reaction, this is a redox reaction. The definitions of oxidation and reduction can be broadened a bit using oxidation numbers: Oxidation is an increase in oxidation number; reduction is a decrease in oxidation number. The gain in oxidation number occurs because electrons are lost during oxidation; the gain of electrons during reduction causes a decrease in the oxidation number. This can be shown by using so-called half-reactions for each process.
Oxidation half-reaction: Na → Na + + e −
Reduction half-reaction: Cl 2 + 2 e − → 2 Cl −
Notice in the balanced equation that two moles of Na were used to react with the two moles of chlorine atoms in one mole of Cl 2 . Each mole of Na lost one mole of electrons; each mole of chlorine atoms gained a mole of electrons. Two moles of electrons were transferred to form two moles of NaCl. The overall reaction is the sum of the two half-reactions; the moles of electrons cancel, and the sodium ions and chloride ions combine to form sodium chloride. Note that the sum of the oxidation numbers in sodium chloride is zero: (+1) + (−1) = 0.
Oxidation half-reaction: 2 Na → 2 Na + + 2 e −
Reduction half-reaction: Cl 2 + 2 e − → 2 Cl −
Overall reaction: 2 Na + Cl 2 → 2 NaCl
Oxidation-reduction reactions, even complex ones, can be balanced using either the half-reaction method or the oxidation number method. The half-reaction method will be discussed first, using the reaction of iron with chlorine to produce iron chloride.
Fe + Cl 2 → FeCl 3 (unbalanced equation)
Half-Reaction Method
Step 1. Divide the reaction into two half-reactions; one corresponding to oxidation, the other, reduction.
Oxidation: Fe → Fe 3+
Reduction: Cl 2 + → Cl −
Step 2. Balance each half-reaction for mass and then charge. The iron half-reaction is balanced with respect to mass because there is one iron on each side. However, the charge is not balanced; the left side has a charge of zero, the right side has a charge of +3. Charge is balanced by adding three electrons to the right side.
Fe → Fe 3+ + 3 e −
The chlorine half-reaction is unbalanced in terms of mass and charge. Mass balance is achieved by using a coefficient of 2 on the right side.
Cl 2 → 2 Cl −
Charge is then balanced by adding two electrons to the left side.
Cl 2 + 2 e − → 2 Cl −
The two half-reactions indicate that three electrons are lost per Fe atom during oxidation, and that two electrons are gained as each Cl 2 molecule is reduced.
Step 3. Combine the two half-reactions in such a way as to balance the electrons lost and gained. The oxidation half-reaction lost three electrons; the reduction half-reaction gained two electrons. Therefore, to balance electrons lost and gained, multiply the oxidation half-reaction by 2 and the reduction half-reaction by 3. Add the resulting half-reactions to get the final balanced equation for the formation of FeCl 3 . Note that, in doing so, the electrons cancel (as they should if the final equation is balanced).
2 [Fe → Fe 3+ + 3 e − ] → 2 Fe → 2 Fe 3+ + 6 e −
3 [Cl 2 + 2 e − → 2 Cl − ] → 3 Cl 2 + 6 e − → 6Cl −
The Fe 3+ and Cl − ions combine to form FeCl 3 and the overall balanced equation is
2 Fe + 3 Cl 2 → 2 FeCl 3
The half-reaction method can be applied to more complex redox reactions, such as the reaction of permanganate ion, MnO 4 − , with Fe 2+ in acidic solution.
MnO 4 − ( aq ) + Fe 2+ ( aq ) → Mn 2+ ( aq ) Fe 3+ ( aq ) (unbalanced equation)
Step 1.
Oxidation: Fe
2+
(
aq
) → Fe
3+
(
aq
)
Reduction: MnO
4
−
(
aq
) → Mn
2+
(
aq
)
Step 2. Mass and charge balance are achieved this way:
The iron is balanced by adding one electron on the right
Fe 2+ ( aq ) → Fe 3+ ( aq ) e −
To balance oxygen, we use H 2 O on the right side; to balance hydrogen, we use H + on the left side (recall that the reaction is taking place in acidic solution)
8 H + ( aq ) + MnO 4 − ( aq ) → Mn 2+ ( aq ) + 4 H 2 O ( l )
The reduction half-reaction has a net charge of +7 on the left [(8+) + (−1)] and +2 on the right [(+2) + 0]. Adding 5 electrons to the left side balances the charge.
8 H + ( aq ) + MnO 4 − ( aq ) + 5 e − → Mn 2+ ( aq ) + 4 H 2 O ( l )
Step 3. Equalize the electrons transferred. Multiply the oxidation half-reaction by 5. Add the half-reactions, canceling the electrons.
5 Fe 2+ ( aq ) → 5 Fe 3+ ( aq ) + 5 e −
8 H + ( aq ) + MnO 4 − ( aq ) + 5 e − → Mn 2+ ( aq ) + 4 H 2 O( l )
Balanced equation: 5 Fe 2+ ( aq ) + 8 H + ( aq ) + MnO 4 − ( aq ) → 5 Fe 3+ ( aq ) + Mn 2+ ( aq ) + 4 H 2 O ( l )
Oxidation Number Method
MnO 4 − ( aq ) + Fe 2+ ( aq ) + H + ( aq ) → Mn 2+ ( aq ) + Fe 3+ ( aq ) H 2 O ( l ) (unbalanced equation)
As in the half-reaction method, H 2 O is used to balance oxygen, and H + is used to balance hydrogen.
Step 1. Identify the oxidation number of each element on each side of the equation. Determine which has undergone oxidation and which has undergone reduction. This is indicated in Table 1.

| Element | Oxidation Number as Reactant | Oxidation Number as Product | Change in Oxidation Number | Oxidation or Reduction |
| Mn | +7 | +2 | Decrease by 5 | Reduction |
| O | −2 | −2 | 0 | Neither |
| Fe | +2 | +3 | Increase by 1 | Oxidation |
| H | +1 | +1 | 0 | Neither |
Step 2. Use coefficients so that the total increase in oxidation number equals the total decrease. In this case, the total decrease is 5 (Mn +7 becomes Mn 2+ ), and the total increase must also be 5; each iron must be multiplied by 5: (5 Fe 2+ becomes 5 Fe 3+ ). Balance hydrogen and oxygen in the usual manner. The balanced equation is
MnO 4 − ( aq ) + 5 Fe 2+ ( aq ) + 8 H + ( aq ) → Mn 2+ ( aq ) + 5 Fe 3+ ( aq ) + 4 H 2 O ( l )
SEE ALSO Chemical Reactions ; Inorganic Chemistry ; Mole Concept ; Organic Chemistry .
Conrad L. Stanitski
Bibliography
Daub, G. William, and Seese, William S. (1996). Basic Chemistry , 7th edition. Upper Saddle River, NJ: Prentice Hall.
Ebbing, Darrell D., and Wentworth, R. A. D. (1998). Fundamentals of Introductory Chemistry , 2nd edition. Boston: Houghton Mifflin.
Goldberg, David E. (2001). Fundamentals of Chemistry , 3rd edition. Boston: McGraw-Hill.
Myers, R. T.; Oldham, K. B.; and Tocci, S. (1999). Holt Chemistry: Visualizing Matter , 2nd edition. Austin, TX: Holt, Rinehart and Winston.
Internet Resources
Bellevue Community College, Science Division. "Balancing Chemical Equations." Available from http://www.scidiv.bcc.ctc.edu/wv/6/0006-002-balancing.html .
New Traditions Project. Establishing New Traditions: Revitalizing the Chemistry Curriculum. "Balancing Chemical Equations." Available from http://newtraditions.chem.wisc.edu/FPTS/fbeqns/chemeqnf.htm .