Epinephrine

Epinephrine, also known as adrenalin , is a hormone that is responsible for the "fight or flight" reaction in mammals. Chemically, it mobilizes the body's defense system, inducing the release into the blood of large amounts of glucose from stores in the liver and muscles. This burst of energy is the familiar "adrenalin rush" one experiences when frightened or excited. In some tissues, epinephrine also acts as a neurotransmitter, conveying signals between adjacent nerve cells.
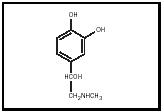
Epinephrine (see Figure 1) is synthesized in several steps from either phenylalanine or tyrosine (both amino acids). Two adjacent hydroxyl groups are placed on the aromatic ring, leading to the ring structure called catechol. These hydroxylations form the intermediate L-dopa, which in turn is converted to dopamine (a neurotransmitter), norepinephrine (also a neurotransmitter), and finally epinephrine. Epinephrine together with norepinephrine and dopamine make up the family of biogenic amines called catecholamines.
Nerve signals to the adrenal gland activate the conversion of stores of norepinephrine to epinephrine and its release into the bloodstream. The fight or flight reaction includes increased blood glucose, increased vasoconstriction in certain parts of the body, and increased heart rate. At the cellular level, epinephrine binds to liver and muscle cells at specific receptors on the outside surface of cell membranes. Such a receptor then activates a series of enzymatic reactions inside the cell, culminating in the synthesis of large amounts of cyclic adenosine monophosphate (cAMP). Epinephrine cannot cross the cell membrane, so its hormonal signal is transmitted inside the cell via cAMP, acting as a second messenger (epinephrine being the first messenger). CyclicAMP switches on a cascade of enzymes—mostly kinases that place a phosphate group at specific sites on other proteins or enzymes. These phosphorylations serve to activate (or in some cases inhibit) enzymatic reactions. The end result is the activation of glycogen phosphorylase, an enzyme that breaks down glycogen into its glucose units, and the release of glucose into the bloodstream.
The neurotransmitter action of epinephrine is terminated by reuptake into the neuron that released it, or breakdown to inactive metabolites by the enzymes catechol-O-methyl transferase (COMT) and monoamine oxidase (MAO). The second messenger effects inside the cell are terminated by enzymes that break down cAMP, and by phosphatases that reverse the action of the kinases by removing phosphates.
Epinephrine also acts at a crucial regulatory step in the synthesis of fatty acids. The activity of the first enzyme in fatty acid synthesis, acetyl-coenzyme A (AcCoA) carboxylase, is regulated by phosphorylation . The phosphorylated enzyme is inactive (and subsequent fatty acid synthesis is halted), whereas the dephosphorylated enzyme is active. Epinephrine, through the second messenger cAMP, prevents the dephosphorylation of AcCoA carboxylase, rendering it inactive and halting the synthesis of fatty acids. Indeed, during the fight or flight reaction, the organism needs to release energy in the form of glucose and fatty acids rather than store energy as glycogen or fat.
Clinically, epinephrine plays a lifesaving role in countering the effects of anaphylactic shock. Histamines released in large amounts upon the body's exposure to an allergen (bee stings in certain individuals, for instance) can constrict smooth muscle, including that in the airway passages. Epinephrine does the opposite: It relaxes smooth muscle, though at different receptors. Its effects on heart muscle (increasing the heart rate) can be used as a life-saving measure when a patient's heart has stopped. Epinephrine is also used in conjunction with local anesthetics such as lidocaine. By constricting blood vessels near the site of the injection, it keeps the anesthetic from diffusing away from the site.
SEE ALSO Kinase .
C. Larry Bering
Bibliography
Devlin, Thomas M., ed. (2002). Textbook of Biochemistry: With Clinical Correlations, 5th edition. New York: Wiley-Liss.
Marieb, Elaine N. (2001). Human Anatomy and Physiology, 5th edition. New York: Addison Wesley Longman.
Voet, Donald, and Voet, Judith G. (1995). Biochemistry, 2nd edition. New York: Wiley.
Comment about this article, ask questions, or add new information about this topic: