Enzymes

Enzymes are (mostly) proteins that catalyze biochemical reactions; that is, they increase the rate of a reaction but are not used up in the process. Their importance to life is underscored by the fact that many severe or fatal genetic diseases involve a missing or defective enzyme. Enzymes can also provide targets, with hitting the target the strategy for attacking disease-causing bacteria or viruses. One could design a drug that attaches to or occupies the active site of a target organism's enzyme. For example, penicillin destroys an enzyme crucial to the synthesis of bacterial cell walls. In the treatment of AIDS, HIV protease inhibitors target the viral enzyme protease.
Properties and Mechanism
In some cases, the increase in the rate of an enzyme-catalyzed reaction versus the uncatalyzed rate is a millionfold. As soon as one reaction has been catalyzed, the enzyme is available for another round of catalysis —a phenomenon known as turnover. Enzymes operate near physiological temperature and pH, and they are also highly specific in their actions—for example, an enzyme called hexokinase will place a phosphate group only onto the sixth carbon of a D-glucose molecule. The enzyme has no activity toward L-glucose, and reduced activity toward other D-sugars. Enzymes can also be regulated so that they are switched on only when they are needed by the cell. They may consist of a single polypeptide chain of amino acids (RNAse contains 124 amino acids), or they may require an additional chemical called a coenzyme. Many of the vitamins as well as several metals act as coenzymes.
A study of enzyme catalysis is a study of kinetics, which asks the question "how fast?" However, enzymes cannot alter the outcome or direction of a reaction. For instance, if one were to add a small amount of sodium chloride to a large volume of water, we know that the end result will be that the salt will dissolve in the water. However, the time dissolution takes depends on a number of factors: What is the temperature?; Is it being stirred? This is kinetics. We also know that a swinging pendulum will eventually come to rest at its equilibrium point, which in this case is its pointing straight down toward the center of Earth. Kinetics describes only the time it takes to reach that point. Enzymes cannot alter the equilibrium point of a reaction, only the time it takes to get there.
To proceed to products, reactants must come together with sufficient energy to overcome an energy barrier known as the energy of activation. The apex of this barrier represents the transition state between reactants and products. Enzymes act to lower the energy of activation by stabilizing (lowering the energy of) the transition state.
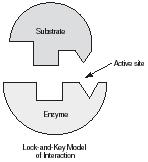
Mechanistically, an enzyme will bind the reactant, called the substrate, at a very specific site on the enzyme known as the active site. This resulting enzyme–substrate complex (ES), described as a lock-and-key mechanism, involves weak binding and often some structural changes—known as induced fit—that assist in stabilizing the transition state. In the unique microenvironment of the active site, substrate can rapidly be converted to product resulting in an enzyme product (EP) complex that then dissociates to release product.
Nomenclature
Enzyme nomenclature has historically been at the whim of the discoverer of the enzyme. Many enzyme names give clues to their actions or to where they are found. The meat tenderizer papain can be obtained from the papyrus plant. Pepsin is a digestive enzyme found in the stomach. Lysozyme acts to lyse bacterial cell walls. Most enzymes are now named by their function along with the suffix "–ase." For instance, ethanol is metabolized in the liver by alcohol dehyrogenase. Phosphates are removed from molecules by phosphatases. An international body known as the Enzyme Commission (EC) has assigned numerical designations, called EC numbers, to enzymes. The EC has listed six categories based on type of activity (oxidation-reduction, hydrolysis, etc.), along with several subcategories, into which enzymes are placed. For example, alcohol dehydrogenase is EC 1.1.1.1.
Enzyme Kinetics
The rate of an enzyme-catalyzed reaction varies with the substrate concentration. At very low substrate concentrations, the rate will be directly proportional to the concentration of the substrate and will exhibit first-order kinetics. However, at very high substrate levels, all of the active sites are occupied and substrate molecules must wait their turn, just as the traffic at a line of tollbooths depends on how fast the cars can pass through the gates. This represents saturation, and the reaction rate is at its maximum and is designated V max .
Other factors that influence enzyme activity include pH and temperature. Most mammalian enzymes operate maximally at around physiological pH and body temperature. There are several exceptions. The stomach digestive enzyme pepsin works best at around pH 2.0, the approximate pH of the stomach. Bacteria found in hot springs have enzymes that operate at or near the boiling point of water. In most cases, however, extremes of pH or temperature will destroy enzymes in an enzyme-unfolding process known as denaturation. Only one, very precise, three-dimensional configuration or fold of the chain of amino acids is functional. Denatured or unfolded proteins are inactive. Denaturation can be observed when an egg white is cooked: The proteins in the egg white unfold and form a gel-like aggregate with other unfolded proteins. The same occurs when bacteria spoil milk by lowering its pH sufficiently to unfold and curdle milk proteins.
RNA as an Enzyme
Although enzymes are considered to be proteins, enzyme activity has recently been found in ribonucleic acid (RNA) in certain organisms. These "ribozymes" may yield clues to the origins of life on Earth. DNA needs enzymes to replicate, whereas enzymes need the instructions of DNA. This represents a "chicken-and-egg" question that has stumped researchers. Early life may have used RNA that was able to catalyze its own replication.
SEE ALSO Catalysis and Catalysts ; Coenzyme ; Denaturation ; Hydrogen ; Inhibitors ; Kinetics ; Proteins ; Ribonucleic Acid .
C. Larry Bering
Bibliography
Campbell, Mary K. (1999). Biochemistry, 3rd edition. New York: Saunders.
Voet, Donald, and Voet, Judith G. (1995). Biochemistry, 2nd edition. New York: Wiley.
Comment about this article, ask questions, or add new information about this topic: