Disaccharides

Sucrose, or table sugar, is the most common disaccharide. Although the term "sugar" is commonly used to refer to sucrose, sucrose is only one of a large group of sugars. Disaccharides are carbohydrates containing two monosaccharides linked by a glycosidic bond. Glycosidic bonds form when the anomeric carbon of one sugar reacts with a hydroxyl group belonging to a second sugar. Sugars with free anomeric carbons can reduce ferric (Fe 3+ ) and cupric (Cu 2+ ) ions, and are called reducing sugars. Anomeric carbons involved in glycosidic bonds are nonreducing. In general, disaccharides and polysaccharides contain both reducing and nonreducing sugars. These carbohydrates are represented and their formulas are written from nonreducing end to reducing end.
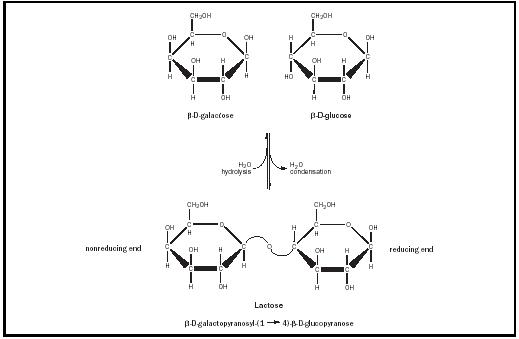
A common disaccharide is lactose, which is found only in milk. Lactose is formed from monosaccharides β -D- galactose and β -D- glucose . The anomeric carbon of the β -D-galactose molecule reacts with the C-4 hydroxyl group of the β -D-glucose molecule to form the glycosidic bond (see Figure 1). The bond is designated a β (1→4) bond, indicating the configuration of the anomeric carbon ( β ), the number of the anomeric carbon (1), and the number of the carbon (of the second sugar) to which it is linked (4). The correct specification of the configuration of the anomeric carbon is critical: an α (1→4) linkage is not the same thing as a β (1→4) linkage. Lactose is a reducing sugar; the β -D-glucose residue has a free anomeric carbon, and therefore the glucose residue is on the reducing end.
Lactose is hydrolyzed to glucose and galactose in the intestine by the enzyme lactase. People who are lactose-intolerant lack this enzyme. In these people, lactose advances in the digestive tract to the large intestine, where it is fermented by intestinal bacteria to produce large amounts of carbon dioxide and organic acids. Today, people with lactose intolerance can purchase milk in which the lactose has already been hydrolyzed, or can buy lactase supplements.
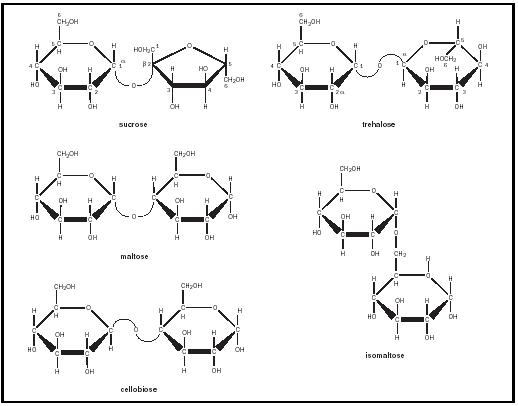
In contrast, most people are fond of and tolerate sucrose (see Figure 2). Sucrose is produced by plant cells; it is one of the major products of photosynthesis . It contains glucose and fructose molecules linked across the anomeric carbons of both (the C-1 of glucose and the C-2 of fructose). The systematic name is α -D-glucopyranosyl-(1→4)- β -D-fructofuranoside. Because it lacks a free anomeric carbon, sucrose is a nonreducing sugar. Sucrose is hydrolyzed in the intestine by the pancreatic enzyme sucrase.
A third prevalent disaccharide is trehalose (see Figure 2). Like sucrose, trehalose is a nonreducing sugar. It is composed of two glucoses in an α (1→1) α linkage. Trehalose is the principal sugar in the hemolymph of insects.
Other disaccharides are the product of the breakdown of larger polysaccharides. Maltose, cellobiose, and isomaltose are all composed of glucose residues (see Figure 2). Maltose and isomaltose both contain α -glucoses: in α (1→4) linkages in the case of maltose, and in α (1→6) linkages in the case of isomaltose. Maltose results from the hydrolysis of starch, and isomaltose from the hydrolysis of dextrans. Cellobiose is composed of β -glucose occurring in β (1→4) linkages. It is a breakdown product of cellulose.
SEE ALSO Carbohydrates .
Stephanie E. Dew
Bibliography
Nelson, David L., and Cox, Michael M. (2000). Lehninger Principles of Biochemistry, 3rd edition. New York: Worth Publishers.
Robyt, John F. (1998). Essentials of Carbohydrate Chemistry. New York: Springer.
Voet, Donald; Voet, Judith G.; and Pratt, Charlotte (1999). Fundamentals of Biochemistry. New York: Wiley.
Internet Resources
American Chemical Society. Division of Carbohydrate Chemistry. Available from http://www.membership.acs.org/C/CARB/ .
International Union of Pure and Applied Chemistry. Available from http://www.chem.qmw.ac.uk/iupac/ .
found in lactose.