Coordination Compounds

Transition metals readily react with halogens to form binary compounds of various colors, for example: green-black ferric chloride (FeCl 3 ), deep blue cobalt chloride (CoCl 2 ), and golden yellow nickel bromide (NiBr 2 ). These compounds dissolve in water to give brightly colored solutions—but of changed colors: yellow solutions (containing Fe 3+ ions), red solutions (Co 2+ ions), and green solutions (Ni 2+ ions). By evaporating the solutions, crystals of these new compounds can be obtained: yellow FeCl 3 · 6H 2 O, red CoCl 2 · 6H 2 O, and green NiBr 2 · 6H 2 O. Addition of ammonia to a green nickel solution changes its color to violet, and the compound NiBr 2 · 6NH 3 can be crystallized. In all cases these beautiful color changes occur because a new chemical species has formed, and there have been changes in the bonding of the nonmetallic substance to the metal ion. Probably the best-known example of vivid color change is the dissolving of anhydrous white cupric sulfate (CuSO 4 ) in water to give a blue solution, containing [Cu(H 2 O) 4 ] 2+ . Addition of ammonia yields the deep blue [Cu(NH 3 ) 4 ] 2+ , which forms crystals that have the formula [Cu(NH 3 ) 4 ]SO 4 · H 2 O.
The neutral molecules NH 3 and H 2 O bond directly to a metal atom by donating a lone pair of electrons to any empty orbital on the metal atom. Anions as well as neutral molecules can bond to a metal atom in this way. These neutral molecules or anions that bond to the metal are called ligands . Further examples of ligands are: Br − , NO 2 − , CO, pyridine, P(CH 3 ) 3 , and O=As(C 2 H 5 ) 3 . Some ligands can donate two pairs of electrons and are termed bidentate , for example, H 2 N–CH 2 –CH 2 –NH 2 (ethylenediamine or en), and O 2 C–CO 2 2− (oxalate anion or ox).
A coordination compound (or coordination complex) consists of a metal cation or neutral atom to which neutral or negatively charged ligands have bonded. The number of ligand atoms to which the metal center is directly bonded is the metal cation's coordination number (c.n.), and this number is always greater than the regular valence or oxidation number (o.n.) of the metal. The coordination complex can be negatively charged, for example, [AuCl 4 ] − , [PtCl 6 ] 2− , [Co(NO 2 ) 6 ] 3− , and [Fe(CN) 6 ] 3− ; neutral, for example, [Fe(CO) 5 ], [Ni(PF 3 ) 4 ], and [Rh(NH 3 ) 3 Cl 3 ]; or positively charged, for example, [Cu(NH 3 ) 4 ] 2+ , [Mn(H 2 O) 6 ] 2+ , and [Pt(NH 3 ) 5 Cl] 3+ . TiCl 4 and UF 6 are neutral molecules (in which o.n. = c.n.), they are not coordination compounds; whereas [AlCl 4 ] − and [FeF 6 ] 3− are coordination complexes in which the coordination number exceeds the oxidation number. The chromate anion, CrO 4 = , is not a coordination complex; the o.n. of the Cr atom is 6, but only four O atoms are bonded to it.
Historical Development
By the mid-1870s Sophus Jørgensen in Denmark had systematized the synthetic methods for preparing the coordination compounds that were known at that time, especially those of cobalt(III). Only in 1893 was the mode of bonding in the complexes established, by Alfred Werner (who was awarded the Nobel Prize in 1913 for this work). Werner concluded that most coordination complexes were essentially octahedral , with six ligands bonded to a central metal ion (more or less, one above, one below, and four in the same plane as the metal ion). He deduced that Pd(II) and Pt(II) complexes were

| NAMING COORDINATION COMPOUNDS BASED ON COLOR | ||
| Color | Formula | Name |
| Yellow | CoCl 3 ·6NH 3 | Luteocobaltic chloride |
| Purple | CoCl 3 ·5NH 3 | Purpurocobaltic chloride |
| Green | CoCl 3 ·4NH 3 | Praseocobaltic chloride |
square-planar, with four ligands bonded to the metal atom. He used the German word Nebenvalenz (meaning secondary valence) for coordination number, and Hauptvalenz (principal valence) for the oxidation number of the metal.
Nomenclature
Naming the compounds was originally based on color. The compound of empirical formula [PtCl 2 (NH 3 ) 2 ], now known to be [Pt(NH 3 ) 4 ][PtCl 4 ], was named Magnus's green salt after its discoverer, Heinrich Gustav Magnus. The modern system, established by the International Union of Pure and Applied Chemistry (IUPAC), assigns names to compounds based on the identity of the metal, its oxidation state, the number and type of ligand (or ligands) attached to it, and the identities of the other cations and anions present. Some examples of IUPAC names are
[Pt(NH 3 ) 2 Cl 2 ] diamminedichloroplatinum(II)
[Co(NH 3 ) 5 Cl]Cl 2 penta-amminecobalt(III) chloride
K 4 [Fe(CN) 6 ] potassium hexacyanoferrate(II)
The problem of nomenclature quickly becomes very great if, for example, a complex contains two different metal atoms linked by bridging groups.
Stereoisomerism
As there is usually more than one way of arranging two or more ligands about a central metal ion, structural isomers , that is, two complexes of the same formula but different chemical and physical properties, are possible.
Geometric isomers. Square- planar complexes [general formula MX 2 B 2 ] and octahedral complexes [general formula MX 2 B 4 ] can exist as geometric isomers, for example, cis and trans [Pd(NH 3 ) 2 Cl 2 ]
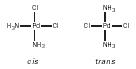
and cis and trans [Pt(NH 3 ) 2 Cl 4 ].
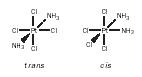
In the case of octahedral complexes, [general formula MX 3 B 3 ,] two isomers exist, facial ( fac ) and meridional ( mer ), for example, fac and mer [Co(NH 3 ) 3 (NO 2 ) 3 ].
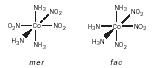
A square-planar complex of formula [MABXY] has three isomers. An example is [PdClBrNH 3 py], whose isomers were prepared by the Russian chemist I. Chernyaev in 1926.
![Three isomers of [PdClBrNH3py]](../images/chfa_01_img0219.jpg)
Polymerization isomers. In the case of octahedral complexes, formula [MX x B b ] n , polymerization isomers are often possible. The value of n is greater than or equal to 1. Several compounds of empirical formula [Co(NH 3 ) 3 (NO 2 ) 3 ] can be isolated.
[Co(NH 3 ) 3 (NO 2 ) 3 ]° ( n = 1)
[Co(NH 3 ) 4 (NO 2 ) 2 ] + [Co(NH 3 ) 2 (NO 2 ) 4 ] − ( n = 2)
[Co(NH 3 ) 6 ] 3+ [Co(NO 2 ) 6 ] 3− ( n = 2)
There are several more possibilities.
Hydration isomers. Hydration isomers exist for crystals of complexes containing water molecules, for example CrCl 3 · 6H 2 O. This compound can exist in three different crystalline forms, in which the number of water molecules directly attached to the Cr 3+ ion differs.
[Cr(H 2 O) 4 Cl 2 ]Cl · 2H 2 O dark green
[Cr(H 2 O) 5 Cl]Cl 2 · H 2 O light green
[Cr(H 2 O) 6 ]Cl 3 gray-blue
In each case, the coordination number of the chromium cation is 6.
Optical isomers. Optical isomers exist for octahedral complexes that do not possess a center of inversion or a mirror plane of symmetry. The complex and its mirror image are not superimposable. One isomer will rotate the plane of polarized light to the left, the other will rotate polarized light to the right. The complexes are said to be chiral and optically active . Some examples are [Co(ox) 3 ] 3− ; cis [Rh(en) 2 Cl 2 ] + ; and cis, cis, cis [PtCl 2 Br 2 (NH 3 ) 2 ].
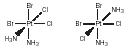
Each of the isomers will react at the same rate with simple monoden-tate ligands such as water. They differ only in their reactivity toward other chiral compounds and toward polarized light.
Stereochemistry
In addition to the common octahedral and square-planar complexes, several other types of complexes (which can be classified according to coordination number) are observed.
Coordination number 2: collinear. Collinear complexes are common in the case of heavy metal cations of d 10 electron configuration. Examples of collinear complexes are [Au(CN) 2 ] − , formed during the extraction of gold from its ore, and [Ag(NH 3 ) 2 ] + , formed when AgCl dissolves in ammonia solution.
Coordination number 3: trigonal planar. Trigonal-planar geometry is quite rare; these complexes are found in instances in which ligands are large and steric repulsions are dominant, for example, [Pt{P(phenyl) 3 } 3 ].
Coordination number 4: tetrahedral. Tetrahedral complexes are common for d 10 metal ion species such as Zn 2+ and Ga 3+ , and d 7 species, such as Co 2+ . Examples of tetrahedral complexes are [ZnCl 2 (pyridine) 2 ]; [GaCl 4 ] − ; and [CoCl 2 (4–methylpyridine) 2 ], which is deep blue.
Coordination number 5: trigonal-bipyramidal and square-pyramidal. Trigonal-bipyramidal and square-pyramidal geometries are common in the case of complexes of metal ions of coordination number 5. Examples of trigonal-bipyramidal complexes are [CuCl 5 ] 3− , [Fe(CO) 3 (PF 3 ) 2 ], and [Mn(CO) 4 NO]. Examples of square-pyramidal complexes are [Ni(CN) 5 ] 3− and [CoCl(dppe) 2 ] + .
Coordination numbers 7, 8, and 9: various. Further types of coordination geometry exist for large cations, especially those of the 3+ lanthanide cations, for example, [Ho(acac) 3 H 2 O], [NbF 7 ] 2− , [Mo(CN) 8 ] 4− , and [ReH 9 ] 2− .
Carbonyls and Organometallic Compounds
Carbonyl compounds and organometallic compounds are two groups of coordination compounds in which carbon atoms are bonded to the metal center. In neutral carbonyls, carbon monoxide molecules are bonded to the metal atom, which is often in oxidation state zero . Examples of neutral carbonyl compounds are [Ni(CO) 4 ] (tetrahedral); [Fe(CO) 5 ] (trigonalbipyramidal); and [Mo(CO) 6 ] (octahedral).
dppe = (C 6 H 5 ) 2 PCH 2 CH 2 P(C 6 H 5 ) 2 ; a bidentate neutral molecule that coordinates through the two phosphorus atoms.
Probably the most famous of the organometallic compounds is the exceedingly stable orange ferrocene, [Fe(C 5 H 5 ) 2 ]. Its discovery in 1951 triggered an explosion in coordination chemistry , and the subsequent preparation of thousands of new organometallic compounds. Ferrocene consists of two planar, five-membered rings sandwiching an iron atom between them. Moreover, the bonding in organometallic compounds follows the same rules as the bonding in the carbonyls. The metal atom accepts electrons from the ligands until it achieves a valence electron count of 18 (the number of valence electrons in an atom of the nearest inert gas). This is called the Effective Atomic Number (EAN), or eighteen-electron rule , first stated by Nevil Sidgwick in 1927 for the metal carbonyls in which each CO group donates two electrons to the metal atom to form an M–C bond. Some examples of the rule are: Ni(CO) 4 , atomic number of Ni = 28, atomic number of Kr = 36, 8 electrons are required, thus 4 CO groups are attached in a tetrahedral arrangement; Re 2 (CO) 10 , atomic number of Re = 75, atomic number of Rn = 86, 11 electrons are required for each Re atom. Five CO groups contribute ten electrons, the eleventh comes from sharing a pair of electrons in a Re–Re metal–metal sigma bond, giving octahedral geometry about the Re atom.
In ferrocene, each cyclopentadienyl ring is considered to donate five electrons to the iron atom, atomic number = 26, giving a total of 10 + 26 = 36 electrons, the atomic number of krypton. Similarly, bonding in the compound [Mn(CO) 3 (C 5 H 4 –CH 3 )] obeys the EAN eighteen-electron rule.
![[Mn(CO)3 (C5H4 CH3)]](../images/chfa_01_img0221.jpg)
The atomic number of Mn = 25; 25 + 5 + (3 × 2) = 36 electrons: the rule is satisfied. This mixed cyclopentadienyl carbonyl complex is of importance as an antiknock compound in gasoline.
Chelating Ligands
EDTA. The bidentate ligands ethylenediamine, oxalate anion, and acetylacetonate anion form chelate complexes of enhanced stability, due to the formation of inflexible five- or six-membered rings with the metal.
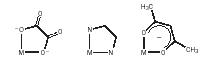
The compound ethylenediaminetetraacetic acid, on deprotonation, yields the hexadentate tetraanion ligand EDTA, which forms remarkably stable complexes by simultaneously bonding through the two nitrogens and four oxygens, one each from the four acetate groups. EDTA is used in toilet soap as a water-softener, in bread as a preservative, and in underarm deodorants as a deodorizer. The magnesium derivative [MgH 2 EDTA] is used to treat patients suffering from heavy metal poisoning. EDTA removes the active cation (M 2+ or M 3+ ) by "engulfing" it to form a water-soluble octahedral complex, which is readily excreted .
![Chiral complex [MEDTA]](../images/chfa_01_img0223.jpg)
The anion D2EHPA, derived from di(ethylhexyl) phosphoric acid, forms a strongly held four-membered {M 2+ O 2 P} ring.
Macrocyclic ligands. Cations of the group one metals form stable complexes with macrocyclic polyethers, for example, Na + and "18–crown–6," (–O–CH 2 –CH 2 –) 6 .
![Macrocylic complex [Na+ 18-crown-6]](../images/chfa_01_img0224.jpg)
The ultimate in encapsulation of a metal cation occurs by ligands termed cryptands , for example, N(CH 2 –CH 2 –O–CH 2 –CH 2 –O–CH 2 –CH 2 ) 3 N, which completely encapsulates the cation; the complex with the K + cation is 1,000 times more stable than the corresponding Na + complex. The preparation and study of these compounds, by Donald J. Cram, Jean-Marie Lehn, and Charles J. Pedersen, earned them the Nobel Prize in chemistry in 1987.
Bonding and Stability
The strength of the metal–ligand bond ranges from the weak ion-dipole interaction between Mg 2+ and the O atom of the water molecule in [Mg(H 2 O) 6 ] 2+ (in Epsom salts), to the very strong bonding in the [Fe(CN) 6 ] units in the pigment Turnbull's blue, to the metal–C covalent bonds in organometallic compounds. The colors and magnetic properties of transition metal complexes can be explained by crystal field theory, while the metal–ligand covalent bonding is described by the molecular orbital approach.
The metal atom in the square-planar complexes of Pd(II), Pt(II), Rh(I), Ir(I) has only sixteen electrons in its valence orbitals. These complexes are easily oxidized by the addition of oxygen or halogens to yield an octahedral complex that obeys the EAN (eighteen-electron) rule. When heated, the 18-electron complex loses the diatomic molecule and the metal is reduced to the 16-electron complex. This cycle is important in homogeneous catalysis .
Homogeneous Catalysis
Coordination complexes serve as catalysts in several important industrial reactions. In the Oxo reaction, Co 2 (CO) 8 reacts with H 2 to form HCo(CO) 4 , which catalyzes the addition of H 2 and CO to olefins to give aldehydes. Wilkinson's catalyst , [RhCl{P(phenyl) 3 } 3 ], is a catalyst used for the hydrogenation of alkenes, especially important in the manufacture of pharmaceuticals because it is very selective and is effective under mild ambient comditions. Polyethylene is produced from ethylene at low pressure by using TiCl 4 /Al(C 2 H 5 ) 3 as a catalyst; for developing this process, Karl Ziegler and Giulio Natta earned a Nobel Prize in 1963.
Extractive Metallurgy
The extraction and purification of the valuable metals by hydrometallurgical processes are totally dependent on the formation of stable coordination compounds. A few examples are
palladium: trans [Pd(NH 3 ) 2 Cl 2 ]
platinum: [PtCl 6 ] 2−
gold: [Au(CN) 2 ] − and [AuCl 4 ] −
uranium: [UO 2 (SO 4 ) 3 ] 4−
cobalt: [Co(D2EHPA) 2 ]
The neutral cobalt D2EHPA complex is unique in its being soluble in kerosene, a property that makes possible the industrial solvent extraction process.
Bioinorganic Systems
Coordination compounds play important roles in nature. Chlorophyll , which is involved in photosynthesis in plants, is a coordination complex of magnesium. Hemoglobin, the oxygen transporter in the human body, is a coordination complex of iron. Vitamin B 12 , necessary for the prevention and cure of pernicious anemia, is a coordination complex of cobalt. In all three compounds, the metal ion is in an approximately octahedral environment, its coordination number is 6, and bonded to it are the four nitrogen atoms of a planar porphyrin -like ring. The basic planar ring structure is closely related to that the extremely stable blue pigment, Cu(II)phthalocyanine.

All metals will form coordination compounds. The stability of a coordination compound depends on the nature of the ligands and the atomic number and oxidation state of the metal.
SEE ALSO Bonding ; Chemotherapy ; Werner, Alfred .
Michael Laing
Bibliography
Bailar, John C., ed. (1956). The Chemistry of the Coordination Compounds. New York: Reinhold.
Cotton, F. Albert, and Wilkinson, Geoffrey (1988). Advanced Inorganic Chemistry , 5th edition. New York: Wiley.
Douglas, Bodie E.; McDaniel, Darl H.; and Alexander, John J. (1994). Concepts and Models of Inorganic Chemistry , 3rd edition. New York: Wiley.
Huheey, James E.; Keiter, Ellen A.; and Keiter, Richard L. (1993). Inorganic Chemistry , 4th edition. New York: Harper Collins.
Jones, Mark M. (1964). Elementary Coordination Chemistry. Englewood Cliffs, NJ: Prentice Hall.
Kauffman, George B., ed. (1968). Classics in Coordination Chemistry, Part 1. New York: Dover.
Kauffman, George B. (1994). Coordination Chemistry: A Century of Progress. Washington, DC: American Chemical Society.
Kettle, Sidney F. A. (1996). Physical Inorganic Chemistry. Sausalito, CA: University Science.
Moeller, Therald (1982). Inorganic Chemistry. New York: Wiley.
Nicholls, David (1974). Complexes and First-Row Transition Elements. London: Macmillan.
Werner, Alfred (1911). New Ideas in Inorganic Chemistry , 2nd edition, translated by E. P. Hedley. London: Longmans, Green & Co.
Wilkinson, Geoffrey; Gillard, Robert D.; and McCleverty, Jon A., eds. (1987). Comprehensive Coordination Chemistry. London: Pergamon.