Base Pairing
James Watson and Francis Crick proposed the molecular structure of deoxyribonucleic acid ( DNA ) in 1952. Gathering a number of experimental findings on DNA, including x-ray diffraction patterns of DNA fibers, they proposed that DNA was a double-stranded helical molecule, with its hydrophobic bases occupying the interior of the molecule, and its hydrophilic
deoxyribose and phosphates groups occupying (or being oriented toward) the outer surfaces, interacting with water. The 20-angstrom (7.9 × 10 −8 -inch) diameter of the helix was consistent with the presence of two adjacent strands and supported the hypothesis that a purine base resided on one strand and a pyrimidine base on the equivalent (homologous) site of the complementary strand.
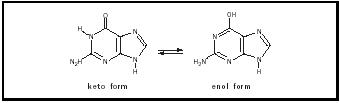
Biochemist Erwin Chargaff had shown that the numbers of adenine and thymine units found in DNA were identical, and likewise for guanine and cytosine units. The ways in which the individual bases in the complementary A–T and G–C base pairs interacted to produce this one-to-one correspondence were not understood. The heterocyclic bases contain functional groups : ring nitrogens, carbonyl groups, and exocyclic amino groups that define much of the character of the bases. Interestingly, each base can occur in two structural forms, called tautomers. For example, the tautomeric pair that describes guanine, referred to as the keto and the enol forms, is shown in Figure 1. They are in equilibrium , with the keto form being favored by a factor of (approximately) 1,000 to 1. Once Watson and Crick had determined the correct tautomeric forms for each base, the nature of the phenomenon of pairing became clear.
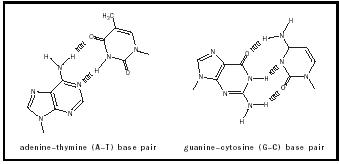
A G–C base pair contains three specific hydrogen bonds within itself, whereas an A–T base pair contains two hydrogen bonds. (See Figure 2.) It is important to note that the presence of an incorrect tautomeric form in DNA would engender very different hydrogen bonding interactions, and the structural complementarity of base pairs would, in this instance, not exist. This simple yet elegant complementarity of the A–T and G–C base pairs also provides a mechanism to ensure that the proper bases are incorporated
into DNA during DNA replication.
SEE ALSO DNA Replication ; Watson, James Dewey .
William M. Scovell
Bibliography
Adam, R. L. P.; Knowler, J. T.; and Leader, D. P. (1992). The Biochemistry of Nucleic Acids , 11th edition. New York: Chapman and Hall.
Garrett, Reginald H., and Grisham, Charles M. (1999). Biochemistry , 2nd edition. Fort Worth, TX: Saunders College Publishers.
Comment about this article, ask questions, or add new information about this topic: