Leo Baekeland
AMERICAN CHEMIST AND INVENTOR
1863–1944
Born in St. Martens-Latem, Belgium, Leo Hendrick Baekeland was the son of a cobbler (Karel Baekeland) and a housemaid (Rosalia Merchie). He earned a B.S. in 1882 and a D.Sc. in 1884, with the highest honors, in organic chemistry from the University of Ghent. He joined the faculty at Ghent, which was then a leading center for the study of coal tar compounds. Interested in becoming an inventor, Baekeland used a traveling scholarship to visit the United States in 1889 (the year he married Celine Swart). His interest in photographic development brought him into contact with Richard Anthony of E. & H. T. Anthony and Company, who recruited Baekeland to join his American-based photographic company.
After two years Baekeland became an independent consultant, but he had little money and few prospects. Nevertheless, after experimenting with silver chloride emulsions, he developed a high-quality photographic printing paper, called Velox, sensitive enough to be used with artificial light. In 1899 the Eastman Kodak Company (located in Rochester, New York) bought the rights to Velox from Baekeland and his partner Leonard Jacobi for $750,000. This product represented a great leap forward in modern photographic technology.
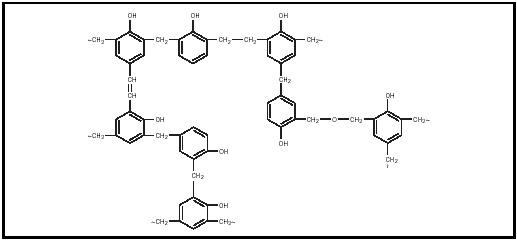
At his home laboratory in Yonkers, New York, Baekeland then returned to his early interest in resin chemistry. Reacting phenol and phenol derivatives with formaldehyde , he learned enough about controlling the aldehyde -phenol ratio with acids and alkalis to synthesize several resins. Most notable was the phenol-formaldehyde polymer resin (Figure 1) he produced with an alkali catalyst . Baekeland developed high pressure and high temperature techniques that greatly improved the molding of this plastic, which he named Bakelite and patented in 1909. It was a superhard, lightweight, insoluble plastic with a tensile strength of 7,000 pound force per square inch (psi). Baekeland claimed to have synthesized the first true plastic.
In 1910 Baekeland founded the General Bakelite Corporation in Perth Amboy, New Jersey, which began producing Bakelite on a commercial scale the following year. Bakelite was sold in liquid and powder form for molding to specifications. It quickly gained popularity in a variety of household and industrial uses—such as electrical insulation, billiard balls, tabletops, switchboards, and (later) automobile ignition systems—where it often replaced natural materials or earlier plastics, especially celluloid. By 1939 the factory was producing more than 50 million pounds of Bakelite a year. Baekeland and his firm controlled more than 400 patents. However, competition from major chemical companies was intensifying, and Baekeland's son George, who had worked for the company since 1923, did not wish to run it. So Baekeland, then seventy-five, sold the firm to Union Carbide and Carbon Corporation for roughly $16.5 million.
Baekeland earned many honors and awards, including the Franklin Medal of the Franklin Institute (1940), and the Perkin Medal (1916) and Messel Medal (1938) of the Society of the Chemical Industry. He was also elected president of the Chemists Club of New York (1904); the American Electrochemical Society (1909); the American Institute of Chemical Engineers (1912); and the American Chemical Society (1924). Eccentric in his old age, Baekeland spent much of his time alone, often in Coconut Grove, Florida, although he maintained a healthy correspondence with a number of colleagues on a broad range of subjects. He died in Beacon, New York, on February 23, 1944.
David B. Sicilia
Bibliography
Haynes, Williams (1945–1954). American Chemical Industry. 6 vols. New York: Van Nostrand Reinhold.
Jenkins, Reese (1975). Images and Enterprise. Baltimore, MD: Johns Hopkins University Press.
Kaufman, Morris (1963). The First Century of Plastics. London: Plastics Institute.
Kettering, Charles (1947). "Leo Hendrik Baekeland." National Academy of Sciences, Biographical Memoir 24:281–302.
Comment about this article, ask questions, or add new information about this topic: