Amino Acid
In 1953, Harold Urey and Stanley Miller carried out an amazing experiment in which they produced "molecules of life" from a mixture of gases that they proposed existed in a primordial earth. The experiments simulated what would happen when lightning strikes provided energy for chemical reactions in the atmosphere and suggested a hypothesis for how life might have developed on our planet. Amino acids were the vital molecules that formed in this experiment and supported this hypothesis for the origin of life.
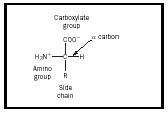
An amino acid is a molecule that contains two functional groups , an amine and a carboxylic acid , as shown in Figure 1. In this illustration there is an additional group called the side chain, designated with an R. The variation seen in naturally occurring amino acids arises from differences in this side chain. The twenty naturally occurring molecules are listed in Table 1. In an aqueous solution , this structure may change so that a proton from the COOH transfers to the NH 2 and a zwitterion is formed. This structure depends on the pH of the solution. Most physiological systems fall into such a pH range so the zwitterion form of amino acids is the most stable form in the human body.
The stereochemistry of amino acids is also an important concept. The carbon atom marked α in Figure 1 is chiral, so an amino acid can be one of two enantiomers. Note that the structure shown in the illustration has the amine group on the right and the carboxyl group at the top. This configuration is designated the L form, and all naturally occurring amino acids have this form.
Amino acids are categorized into three groups based on the nature of the side chain. Nine of the amino acids have side chains that are nonpolar . Almost 50 percent of the amino acids that are present in proteins have nonpolar side chains. The second category of amino acid contains six different molecules that have polar side chains. Finally, a group of five amino acids have side chains that are not only polar, but charged.
The key chemical characteristic of amino acids is that they link together to form proteins. Because the COOH functional group is an acid and the NH 2 functional group is a base, the two ends of amino acids can readily react with each other. Protein synthesis is more complicated than a simple acid-base neutralization, but consider what happens when two amino
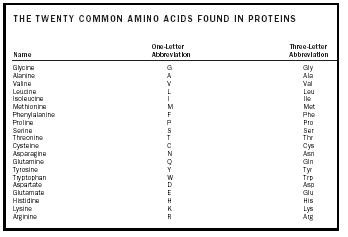
| THE TWENTY COMMON AMINO ACIDS FOUND IN PROTEINS | ||
| Name | One-Letter Abbreviation | Three-Letter Abbreviation |
| Glycine | G | Gly |
| Alanine | A | Ala |
| Valine | V | Val |
| Leucine | L | Leu |
| Isoleucine | I | Ile |
| Methionine | M | Met |
| Phenylalanine | F | Phe |
| Proline | P | Pro |
| Serine | S | Ser |
| Threonine | T | Thr |
| Cysteine | C | Cys |
| Asparagine | N | Asn |
| Glutamine | Q | Gln |
| Tyrosine | Y | Tyr |
| Tryptophan | W | Trp |
| Aspartate | D | Asp |
| Glutamate | E | Glu |
| Histidine | H | His |
| Lysine | K | Lys |
| Arginine | R | Arg |
acids react to form a peptide bond. The new molecule, now a dimer with two amino acid residues still has one end that is an acid and another end that is a base, so it is apparent that the process of forming a peptide bond with another amino acid could be repeated. If many amino acids are strung together, the result is a natural polymer—a protein.
SEE ALSO Proteins ; Urey, Harold ; Zwitterion .
Thomas A. Holme
Bibliography
Barrett, G. C., and Elmore, D. T. (1998). Amino Acids and Peptides. New York: Cambridge University Press.
Cantor, C. R., and Schimmel, P. R. (1980). Biophysical Chemistry, Part I. San Francisco: W.H. Freeman.
Jones, J. (2002). Amino Acid and Peptide Synthesis. Oxford: Oxford University Books.
Comment about this article, ask questions, or add new information about this topic: