Carbohydrates

Carbohydrates are the most abundant natural organic compounds on Earth. The term "carbohydrate" derives from their general formula of C n (H 2 O) n , first determined in the nineteenth century, and indicates that these compounds are hydrates of carbon. Carbohydrates are more specifically defined as polyhydroxy aldehydes or ketones and the products derived from them. Carbohydrates are synthesized via photosynthesis by plants, algae, and some bacteria. Animals feeding on these organisms then use the energy stored in these compounds.
Energy storage is not the only function of carbohydrates. They have a variety of functions in living organisms, including their contribution to the structure of cell walls and their vital role in communication at the site of cell membranes. Carbohydrates form part of the backbone of RNA and DNA molecules, and they are also found linked to proteins and lipids as glycoproteins and glycolipids. The three basic groups of carbohydrates based on size are: monosaccharides , oligosaccharides, and polysaccharides (saccharide from the Greek sakcharon , or "sugar"). The oligosaccharides and polysaccharides are composed of a few and many monosaccharides, respectively. Monosaccharides have two major groups: the aldoses and the ketoses.
Aldoses
The simplest of the aldoses is glyceraldehyde, which is a triose, or three-carbon sugar. Glyceraldehyde has one chiral carbon and therefore two stereoisomers, designated D and L. In nature, only D sugars occur in abundance. Other aldoses can be derived from glyceraldehyde via insertion of additional hydroxy carbons between the carbonyl carbon and the molecule's other carbons. In this way, tetroses, pentoses, and hexoses are formed. Although glyceraldehyde and the tetroses can occur only as simple linear structures,
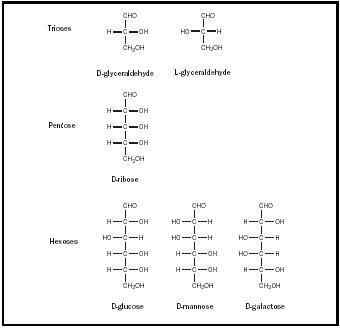
the pentoses and the hexoses can also form rings. The ring formation has important effects on the properties of these molecules.
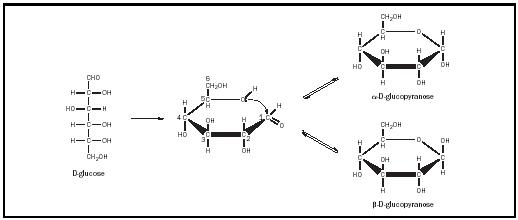
Glucose , an aldohexose, is the most common of the monosaccharides. In various combinations and permutations, it forms starch, cellulose, sucrose (table sugar), and lactose (milk sugar), among other things. When metabolized via the glycolytic pathway, it is the major energy source for many living things. Most commonly, glucose forms a ring, its fifth hydroxyl group reacting with the aldehyde carbonyl group to form a hemiacetal (see Figure 2). As a result of this reaction, the sugar forms a six-membered ring and the carbonyl carbon becomes chiral. The two new stereoisomers of glucose that revolve on the aldehyde carbon are designated α and β and are considered anomers of one another. The now chiral carbon is called the "anomeric" carbon. These six-membered ring structures are called pyranoses, as they resemble the compound pyran. Thus, in its ring forms, glucose is properly designated α -D-glucopyranose, or β -D-glucopyranose.
These pyranose rings are not flat and can assume several different conformations. In general, the most stable conformation is the "chair" conformation, in which the bulky atomic groups (e.g., the hydroxyl and hydroxymethyl groups) are equatorial or within the plane of the ring (see Figure 3). The hydrogens would then be axial or perpendicular to the plane of the ring. Only in β -D-glucopyranose are all of the bulky groups equatorial; thus, β -D-glucopyranose is more stable than α -D-glucopyranose, in which the C-1 hydroxyl group is axial to the ring. The α and β forms of
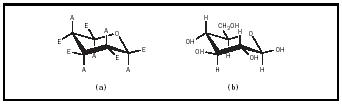
glucose are freely interconvertible in solution. At equilibrium, a solution of glucose contains about two-thirds α -D-glucopyranose, one-third α -D-glucopyranose, and small amounts of the linear and five-membered ring forms of glucose.
Other widely occurring aldoses include mannose, galactose , and ribose (see Figure 1). Mannose and galactose are, like glucose, aldohexoses and can form six-membered rings. Mannose is an important part of the complex sugars, or oligosaccharides, that attach to proteins in the formation of glycoproteins. Galactose combines with glucose to form lactose or milk sugar. Structurally, each of these sugars differs from glucose only in the stereo-chemistry that revolves on one carbon: mannose on C-2 and galactose on C-4. Sugars that differ from one another only in respect to the stereo-chemistry at one carbon are considered epimers of each other. Thus mannose is the C-2 epimer of glucose, and galactose the C-4 epimer. Mannose and galactose are not epimers; they differ from each other in respect to the stereochemistry revolving around two carbons.
Ribose is an aldopentose. It composes the carbohydrate portion of the ribonucleotides that form a cell's RNA. Ribose, like the aldohexoses, can form a ring. However, ribose forms a five-membered ring, called a "furanose" because of its similarity to the compound furan. Once again, there are two possible forms this ring can have: α -D-ribofuranose and β -D-ribofuranose. RNA contains β -D-ribofuranose.
Ketoses
There are fewer ketoses than there are aldoses because ketoses have one less chiral carbon. The most prevalent of the ketoses are dihydroxyacetone, ribulose, xylulose, and fructose (see Figure 4). All four of these sugars are important intermediates in metabolism . Fructose is, along with glucose, part of sucrose or table sugar. Fructose is a ketohexose, and the only one of the four ketoses that can assume a ring structure. Like ribose, fructose forms a five-membered (or furanose) ring and has α and β anomers.
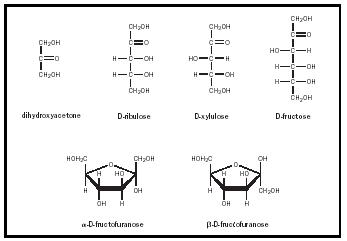
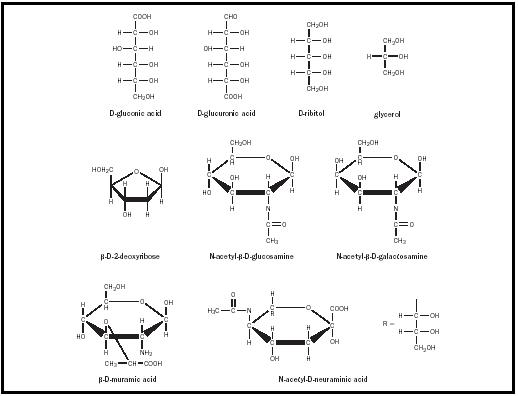
Monosaccharide Derivatives
Monosaccharides undergo incorporation into oligo- and polysaccharides. Individual monosaccharides can also undergo a variety of transformations. One important modification of monosaccharides is the formation of deoxy sugars. The most biologically significant of the deoxy sugars is β -D-2-deoxyribose (see Figure 5), in which the C-2 hydroxyl group of ribose has been replaced with hydrogen. This deoxy sugar is the sugar component of DNA.
In the amino sugars, an amino group replaces one or more of the hydroxyl groups. The most common of these sugars are D-glucosamine and D-galactosamine, both of which have an amino group in place of the hydroxyl group on the second carbon. Often, these amino groups are acetylated to give N-acetyl sugars, such as N-acetylglucosamine and N-acetylgalactosamine. These sugars are important components of larger polysaccharides. Other important amino sugars are muramic acid and N-acetylneuraminic acid, which are components of the oligosaccharides of glycoproteins and glycolipids, and of bacterial cell walls. Muramic acid and N-acetylneuraminic acid are glucosamines, which have been linked at either C-3 or C-1 to three-carbon acids. Muramic acid is formed via an ether linkage between the C-3 of glucosamine and the hydroxyl group of lactic acid. N-acetyl-D-neuraminic acid results from the formation of a C–C bond between the C-1 of N-acetyl-D-mannosamine and the C-3 of phosphoenolpyruvate. This and other derivatives of neuraminic acid are collectively called sialic acids and are widely found in bacteria and animals.
Finally, monosaccharides often form ester linkages with phosphate and sulfate ions. In fact it is rare to find free monosaccharide in cells. Glucose is the only unmodified monosaccharide that exists in substantial quantities in living things, in which it exists primarily extracellularly.
SEE ALSO Chirality ; Glycolysis .
Stephanie E. Dew
Bibliography
Nelson, David L., and Cox, Michael M. (2000). Lehninger Principles of Biochemistry , 3rd edition. New York: Worth Publishers.
Robyt, John F. (1998). Essentials of Carbohydrate Chemistry. New York: Springer.
Voet, Donald; Voet, Judith G.; and Pratt, Charlotte (1999). Fundamentals of Biochemistry. New York: Wiley.
Other Resources
American Chemical Society. Division of Carbohydrate Chemistry. Information available from http://membership.acs.org/C/ .
International Union of Pure and Applied Chemistry. Information available from http://www.chem.qmw.ac.uk/iupac/ .
Comment about this article, ask questions, or add new information about this topic: